Treatment
General
- Treatment intensity level as a proxy for severity of chronic obstructive pulmonary disease: a risk stratification tool. Respir Med. Published online July 31, 2024. doi:10.1016/j.rmed.2024.107742 [MEDLINE]
- Background: Increasing severity of chronic obstructive pulmonary disease (COPD) is associated with increasing risk of poor outcomes. Using health registry data, we aimed to assess the association between treatment intensity levels (TIL), as a proxy for underlying COPD severity, and long-term outcomes
- Methods: Using Danish nationwide registries, we identified patients diagnosed with COPD during 2001-2016, who were alive at index date of 1 January 2017. We stratified patients into exclusive TILs from least to most severe: no use, short term therapy, mono-, dual-, triple therapy, oral corticosteroid (OCS), and long-term oxygen treatment (LTOT). Survival analyses were used to assess 5-year outcomes by TIL
- Results: We identified 53,803 patients with COPD in the study period (median age: 72 years [inter quartile range, 64-80], 48 % male). The three most severe TILs were associated with a significant incremental increase in all-cause mortality with an adjusted hazard ratio (aHR) for triple therapy, OCS and LTOT of 1.44 (95 % CI: 1.38-1.51), 1.67 (95 % CI: 1.59-1.75), and 2.91 (95 % CI: 2.76-3.07) compared with those receiving no therapy as reference. The same pattern was evident for the composite outcome of 5-year mortality or COPD-related hospitalization with an aHR for triple therapy, OCS and LTOT of 2.30 (95 % CI: 2.22-2.38), 2.85 (95 % CI: 2.74-2.96), and 4.00 (95 % CI: 3.81-4.20), respectively
- Conclusion: Increasing TILs were associated with increasing five-year mortality and risk of COPD-related hospitalization. TILs may be used as a proxy for underlying COPD severity in epidemiological studies.
Avoidance of Tobacco Exposure (see Tobacco)
- Recommendation (American College of Chest Physicians/Canadian Thoracic Society Guidelines on the Prevention of COPD Exacerbations, 2015) (Chest, 2015) [MEDLINE]
- Smoking Cessation is Recommended to Decrease the COPD Exacerbation Rate (Grade 2C Recommendation)
- Geographic Disparities by Rural-Urban Status and Drive Time to Care in Tobacco Treatment for COPD. JAMA Netw Open. 2025 Aug 1;8(8):e2528898. doi: 10.1001/jamanetworkopen.2025.28898 [MEDLINE]
- Importance: Many individuals with chronic obstructive pulmonary disease (COPD) who continue to use tobacco do not receive the key intervention of tobacco dependence treatment (TDT). Rurality and drive time to health care services may affect the provision of TDT
- Objective: To examine associations of rurality and drive time to care with provision of TDT among individuals with COPD
- Design, setting, and participants: This retrospective cohort study included individuals with COPD who received care from Veterans Affairs (VA) between January 2012 and December 2019. Administrative data from the Veterans Health Administration were used. Individuals with at least 2 encounters with International Classification Disease codes for COPD and current tobacco use were included. Data analysis was conducted in October 2023
- Exposures: Rural vs urban home address and drive time to closest VA pulmonary specialty care facility using individual geocoded addresses
- Main outcomes and measures: The main outcome was prescription of TDT pharmacotherapy and/or counseling. Multivariable logistic regression models were used to assess associations of rurality and drive time with prescription of TDT
- Results: Among 238 433 individuals with COPD and current tobacco use, the mean (SD) age was 64.1 (9.8) years; 81 189 (93.9%) were male; 2560 (1.1%) were American Indian or Alaska Native, 34 230 (14.3%) were Black or African American, and 185 791 (77.9%) were White. Overall, 97 253 (40.8%) lived in a rural area, and 65 105 (27.4%) had drive times of 61 minutes or longer. Overall, TDT was prescribed to 86 469 individuals (36.3%), but combined pharmacotherapy and counseling only to 10 302 (4.3%). Prescription of any TDT decreased with longer drive times to the closest pulmonary care (eg, 42 324 of 111 126 individuals [38.1%] with drive times ≤30 minutes; 4689 of 14 455 individuals [32.4%] with drive times >120 minutes). In models adjusted for sociodemographic characteristics (age, race and ethnicity, sex, and Area Deprivation Index) and comorbidities, individuals living in a rural area had lower probability of TDT compared with their urban counterparts (34.7% [95% CI, 34.4%-35.0%] vs 37.0% [95% CI, 36.7%-37.2%]). Prescription of TDT steadily decreased from drive time of 30 minutes or less (37.3% [95% CI, 37.0%-37.6%]) to drive times longer than 120 minutes (32.8% [95% CI, 32.1%-33.6%])
- Conclusions and relevance: In this cohort study of individuals with COPD who smoke, the overall provision of TDT-arguably the most important of all COPD interventions-was low. Additionally, rural residence and longer drive time to specialty care were associated with lower likelihood of receiving TDT. These findings highlight the need to address TDT for all individuals with COPD, especially those facing geographic disparities, to improve health outcomes.
Influenza Vaccination (see Influenza Virus)
- Recommendation (American College of Chest Physicians/Canadian Thoracic Society Guidelines on the Prevention of COPD Exacerbations, 2015) (Chest, 2015) [MEDLINE]
- Annual Influenza Vaccination is Recommended to Decrease the COPD Exacerbation Rate (Grade 1B Recommendation)
Pneumococcal Vaccination (see Streptococcus Pneumoniae)
- Clinical Efficacy
- Systematic Review of Pneumococcal Vaccination in COPD (Cochrane Database Syst Rev, 2010) [MEDLINE]
- Polyvalent Pneumococcal Vaccine Had No Impact on Any Outcome in COPD: outcomes studied included risk of pneumonia, COPD exacerbation rate, risk of hospital admission, risk of emergency department visits, all-cause mortality, or death from a cardiorespiratory cause
- Systematic Review of Pneumococcal Vaccination in COPD (Cochrane Database Syst Rev, 2010) [MEDLINE]
- Recommendation (American College of Chest Physicians/Canadian Thoracic Society Guidelines on the Prevention of COPD Exacerbations, 2015) (Chest, 2015) [MEDLINE]
- 23-Valent Pneumococcal Vaccination is Recommended, Although There is No Evidence that it Decreases COPD Exacerbation Rate (Grade 2C Recommendation)
Pulmonary Rehabilitation (see Pulmonary Rehabilitation)
- Clinical Efficacy-General
- Systematic Review of Pulmonary Rehabilitation Following COPD Exacerbation (Cochrane Database Syst Rev, 2011) [MEDLINE]
- Pulmonary Rehabilitation Following COPD Exacerbation Decreased Hospital Admissions and Mortality Rate
- Pulmonary Rehabilitation Following COPD Exacerbation Improved Health-Related Quality of Life
- Pulmonary Rehabilitation Following COPD Exacerbation Improved Exercise Capacity with No Adverse Events Reported
- Study of Effects of Depression on the Completion of Pulmonary Rehabilitation (Respir Med, 2014) [MEDLINE]
- Lower Prevalence of Depressed Mood was a Predictor for Completion of Pulmonary Rehabilitation
- Lower Prevalence of Depressed Mood was a Predictor for Completion of Pulmonary Rehabilitation in Females, But Not in Males
- Greater 6MWT Distance was also an Independent Predictor for Completion of Pulmonary Rehabilitation in Females
- Study of Pulmonary Rehabilitation on Dyspnea in COPD (BMJ Open Respir Res, 2022) [MEDLINE]
- Individualized Home-Based Pulmonary Rehabilitation (8 wks) Improved Both Physical and Affective Components of Dyspnea (at Short-Term and Long-Term)
- Systematic Review of Pulmonary Rehabilitation Following COPD Exacerbation (Cochrane Database Syst Rev, 2011) [MEDLINE]
- Clinical Efficacy-Prevention of Hospitalization for COPD Exacerbation
- Systematic Review and Meta-Analysis of Pulmonary Rehab in the Prevention of Hospitalization for COPD Exacerbation (Chest, 2016) [MEDLINE]; n = 18 studies
- Pulmonary Rehab Did Not Decrease the Rate of Hospitalization for COPD Exacerbation: likely due to the heterogeneous nature of patients included in observational studies and the varying standard of pulmonary rehab programs
- Systematic Review and Meta-Analysis of Pulmonary Rehab in the Prevention of Hospitalization for COPD Exacerbation (Chest, 2016) [MEDLINE]; n = 18 studies
Cost-effectiveness of pulmonary rehabilitation among US adults with chronic obstructive pulmonary disease. JAMA Netw Open. 2022;5(6):e2218189. doi:10.1001/jamanetworkopen.2022.18189
- Recommendation (Joint ACCP/AACVPR Evidence-Based Pulmonary Rehabilitation Guidelines, 2007) (Chest, 2007) [MEDLINE]
- A Program of Exercise Training of the Muscles of Ambulation is Recommended as a Mandatory Component of Pulmonary Rehabilitation for COPD (Grade 1A Recommendation)
- Pulmonary Rehabilitation Improves the Dyspnea in COPD (Grade 1A Recommendation)
- Pulmonary Rehabilitation Improves Health-Related Quality of Life in COPD (Grade 1A Recommendation)
- Pulmonary Rehabilitation Decreases the Number of Hospital Days and Other Measures of Health Care Utilization in COPD (Grade 2B Recommendation)
- Pulmonary Rehabilitation is Cost-Effective in COPD (Grade 2C Recommendation)
- Insufficient Evidence to Determine Whether Pulmonary Rehabilitation Improves Survival in COPD (No Recommendation)
- There are Psychosocial Benefits from Comprehensive Pulmonary Rehabilitation Programs in COPD (Grade 2B Recommendation)
- Six-Twelve Weeks of Pulmonary Rehabilitation Produces Benefits in Several Outcomes Which Decline Gradually Over 12-18 mo (Grade 1A Recommendation)
- Some Benefits (Such as Health-Related Quality of Life) Remain Above Control Levels at 12-18 mo (Grade 1C Recommendation)
- Longer Pulmonary Rehabilitation Programs (Beyond 12 wks) Produce Greater Sustained Benefits than Shorter Programs (Grade 2C Recommendation)
- Maintenance Strategies Following Pulmonary Rehabilitation Have a Modest Effect on Long-Term Outcomes (Grade 2C Recommendation)
- Lower Extremity Exercise Training at Higher Exercise Intensity Produces Greater Physiologic Benefits than Lower Intensity Training in COPD (Grade 1B Recommendation)
- Both Low-Intensity and High-Intensity Exercise Training Produce Clinical Benefits for COPD (Grade 1A Recommendation)
- Addition of a Strength-Training Component to a Program of Pulmonary Rehabilitation Increases Muscle Strength and Muscle Mass (Grade 1A Recommendation)
- Current Evidence Does Not Support the Routine Use of Anabolic Agents in Pulmonary Rehabilitation for COPD (Grade 2C Recommendation)
- Unsupported Endurance Training of the Upper Extremities is Beneficial in COPD and Should Be Included in Pulmonary Rehabilitation Programs (Grade 1A Recommendation)
- Current Evidence Does Not Support the Routine Use of Inspiratory Muscle Training as an Essential Component of Pulmonary Rehabilitation (Grade 1B Recommendation)
- Education (Information on Collaborative Self-Management and the Prevention/Treatment of Exacerbations) Should Be an Integral Component of Pulmonary Rehabilitation (Grade 1B Recommendation)
- Minimal Evidence to Support the Benefits of Psychosocial Interventions as a Single Therapeutic Modality (Grade 2C Recommendation)
- Although No Recommendation is Provided, Since Evidence is Lacking, Current Practice and Expert Opinion Support the Inclusion of Psychosocial Interventions as a Component of Comprehensive Pulmonary Rehabilitation Programs for COPD
- Supplemental Oxygen Should Be Used During Rehabilitative Exercise Training in Patients with Severe Exercise-Induced Hypoxemia (Grade 1C Recommendation)
- Supplemental Oxygen During High-Intensity Exercise Programs in Patients without Exercise-Induced Hypoxemia May Improve Gains in Exercise Endurance (Grade 2C Recommendation)
- As an Adjunct to Exercise Training in Selected Patients with Severe COPD, Noninvasive Ventilation Produces Modest Additional Improvements in Exercise Performance (Grade 2B Recommendation)
- Insufficient Evidence to Support the Routine Use of Nutritional Supplementation in the Pulmonary Rehabilitation in COPD (No Recommendation)
- Pulmonary Rehabilitation is Beneficial for Patients with Some Chronic Respiratory Diseases Other than COPD (Grade 1B Recommendation)
- Although No Recommendation is Provided Since Evidence is Lacking, the Current Practice and Expert Opinion Suggest that Pulmonary Rehabilitation for Patients with Chronic Respiratory Diseases Other than COPD Should Be Modified to Include Treatment Strategies Specific to Individual Diseases and Patients, in Addition to Treatment Strategies Common to Both COPD and Non-COPD Patients
- Recommendation (American College of Chest Physicians/Canadian Thoracic Society Guidelines on the Prevention of COPD Exacerbations, 2015) (Chest, 2015) [MEDLINE]
- In Moderate-Very Severe COPD with a Recent Exacerbation (Within 4 wks), Pulmonary Rehabilitation is Recommended to Decrease the COPD Exacerbation Rate (Grade 1C Recommendation)
- In Moderate-Very Severe COPD with an Exacerbation >4 wks Ago, Pulmonary Rehabilitation is Not Recommended to Decrease the COPD Exacerbation Rate (Grade 2B Recommendation)
Telehealthcare/Telemonitoring
- Clinical Efficacy
- Systematic Review of Telehealthcare in COPD (Cochrane Database Syst Rev, 2011) [MEDLINE]
- Telehealthcare in COPD Had a Possible Impact on the Quality of Life and the Number Emergency Department/Hospital Visits: however, further study is required
- Systematic Review of Telehealthcare in COPD (Cochrane Database Syst Rev, 2011) [MEDLINE]
- Recommendation (American College of Chest Physicians/Canadian Thoracic Society Guidelines on the Prevention of COPD Exacerbations, 2015) (Chest, 2015) [MEDLINE]
- Telemonitoring (as Compared to Usual Care) is Not Recommended to Decrease the COPD Exacerbation Rate (as Assessed by Decreases in Emergency Room Visits, Exacerbations, or Hospitalizations Over a 12 mo Period (Grade 2C Recommendation)
High Altitude (see High Altitude)
- XXXXX
- The effect of chronic altitude exposure on COPD outcomes in the SPIROMICS cohort. Am J Respir Crit Care Med. Published online March 20, 2024. doi:10.1164/rccm.202310-1965OC [MEDLINE]
- Rationale: Individuals with COPD have airflow obstruction and maldistribution of ventilation. For those living at high altitude, any gas exchange abnormality is compounded by reduced partial pressures of inspired oxygen
- Objectives: Does residence at higher-altitude exposure affect COPD outcomes, including lung function, imaging characteristics, symptoms, health status, functional exercise capacity, exacerbations, or mortality?
- Methods: From the SPIROMICS cohort, we identified individuals with COPD living below 1,000 ft (305 m) elevation (n= 1,367) versus above 4,000 ft (1,219 m) elevation (n= 288). Multivariable regression models were used to evaluate associations of exposure to high altitude with COPD-related outcomes
- Measurements and main results: Living at higher altitude was associated with reduced functional exercise capacity as defined by 6MWD (-32.3 m, (-55.7 to -28.6)). There were no differences in patient-reported outcomes as defined by symptoms (CAT, mMRC), or health status (SGRQ). Higher altitude was not associated with a different rate of FEV1 decline. Higher altitude was associated with lower odds of severe exacerbations (IRR 0.65, (0.46 to 0.90)). There were no differences in small airway disease, air trapping, or emphysema. In longitudinal analyses, higher altitude was associated with increased mortality (HR 1.25, (1.0 to 1.55)); however, this association was no longer significant when accounting for air pollution
- Conclusions
- Chronic altitude exposure is associated with reduced functional exercise capacity in individuals with COPD, but this did not translate into differences in symptoms or health status
- Additionally, chronic high-altitude exposure did not affect progression of disease as defined by longitudinal changes in spirometry
- High altitudes and partial pressure of arterial oxygen in patients with chronic obstructive pulmonary disease – A systematic review and meta-analysis. Pulmonology. 2024 Jul 18:S2531-0437(24)00095-3. doi: 10.1016/j.pulmoe.2024.06.002 [MEDLINE]
- Importance: Prior study in healthy subjects has shown a reduction of partial pressure of arterial oxygen (PaO2) by -1.60 kPa/kilometre of altitude gain. However, the association of altitude-related change in PaO2 and altitude-related adverse health effects (ARAHE) in patients with chronic obstructive pulmonary disease (COPD) remain unknown
- Objective: To provide an effect size estimate for the decline in PaO2 with each kilometre of altitude gain and to identify ARAHE in relation to altitude in patients with COPD. www.crd.york.ac.uk/prospero: CRD42020217938
- Data sources: A systematic search of PubMed and Embase was performed from inception to May 30, 2023
- Study selection: Peer-reviewed and prospective studies in patients with COPD staying at altitudes >1500 m providing arterial blood gases within the first 3 days at the target altitude
- Data extraction and synthesis: Aggregate data (AD) on study characteristics were extracted, and individual patient data (IPD) were requested. Estimates were pooled using random-effects meta-analysis
- Main outcome and measures: Relative risk estimates and 95 % confidence intervals for the association between PaO2 and altitude in patients with COPD
- Results: Thirteen studies were included in the AD analysis, of which 6 studies (222 patients, 45.2 % female) provided IPD, thus were included in the quantitative analysis. The estimated effect size of PaO2 was -0.84 kPa [95 %CI, -0.92 to -0.76] per 1000 m of altitude gain (I2=65.0 %, P < 0.001). In multivariable regression analysis, COPD severity, baseline PaO2, age and time spent at altitude were predictors for PaO2 at altitude. Overall, 37.8 % of COPD patients experienced an ARAHE, whereas older age, female sex, COPD severity, baseline PaO2, and target altitude were predictors for the occurrence of ARAHE (area under ROC curve: 0.9275, P < 0.001)
- Conclusions and relevance: This meta-analysis, providing altitude-related decrease in PaO2 and risk of ARAHE in patients with COPD ascending to altitudes >1500 m, revealed a lower altitude-related decrease in PaO2 in COPD patients compared with healthy. However, these findings might improve patient care and facilitate decisions about initiating preventive measures against hypoxaemia and ARAHE in patients with COPD planning an altitude sojourn or intercontinental flight, i.e. supplemental oxygen or acetazolamide.
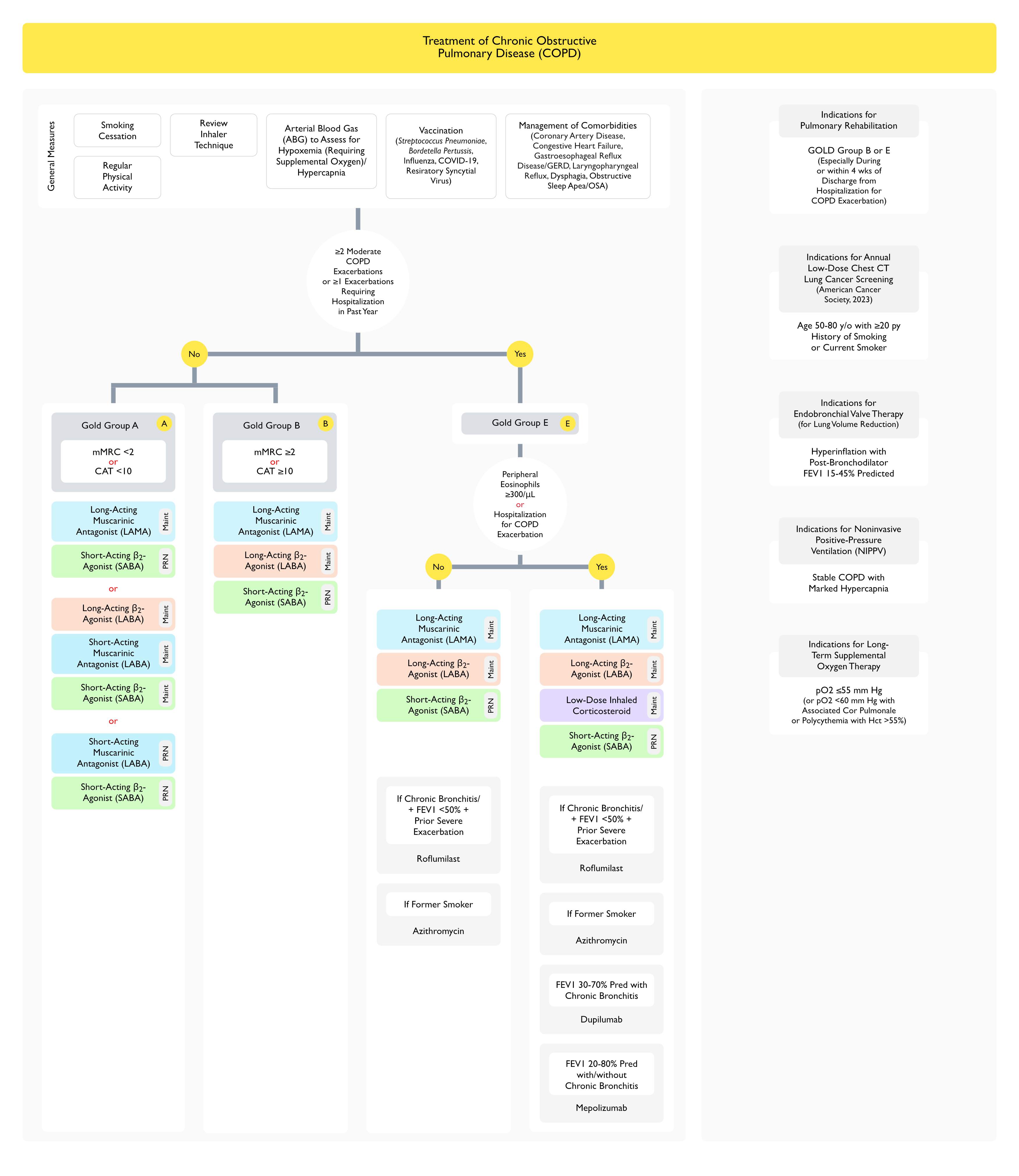
Oxygen (see Oxygen)
Epidemiology
- Long-Term Oxygen Therapy is Used in 1 Million Patients Per Year in the US Via Medicare and Accounts for a Cost of >$2 Billion Per Year (Am J Respir Crit Care Med, 2006) [MEDLINE]: cost appears to be increasing at 13% per year
- Indications: pO2 <55 or pO2 <59 + cor pulmonale or polycythemia
- Physiology: flow is highest during the early part of inspiration (this is when most of the oxygen is delivered to the alveoli)
- Daytime O2: prevents polycythemia/variably decreases PA pressure acutely but, long-term prevents increases in PA pressure/improves survival (when used for at >18 hrs per day, per data from NOTT Trial)/increases exercise endurance/decreases dyspnea
- Nocturnal O2: blunts increases in PA pressures that occur with nocturnal desaturations/inhibits development of polycythemia/prolongs survival
- Nocturnal use alone does not significantly prevent the development of pulmonary hypertension
- Prognostic Factors in COPD Patients on Chronic O2 (Chailleux; Chest, 1996):
- Good Prognosis: stable pCO2 >55 (but rising pCO2 portends poor prognosis, represents progressing cor pulmonale)
- Poor Prognosis: older age/male gender/lower BMI/lower pO2/lower FEV1 (as %pred)
- NOTT Trial: data suggested that 45% of those discharged with oxygen (for pO2 <55) no longer required oxygen when assessed later (1 month) after discharge, therefore reevaluation is necessary
- Transtracheal O2 Delivery: results in decreased oxygen requirements (due to more efficient delivery), decreased work of breathing (possibly due to wash out of anatomic dead space with less CO2 reinspired and possiblt less demanding pattern of breathing), decreased sleep apneas/hypopneas (possibly due to oxygen delivery even during obstructive events or increased mean airway pressure), and decreased dyspnea (unclear if it affects the rate of COPD exacerbations)
- Impact of Oxygen Therapy in COPD with Chronic Hypercapnia: excessive oxygen therapy in the setting of chronic hypercapnia may result in worsened CO2 retention and respiratory failure
- Excess oxygen may blunt the hypoxic ventilatory drive and/or increase physiologic dead space (due to oxygen-induced bronchodilation in poorly-perfused areas of the lung)
Clinical Efficacy
- Multicenter Nocturnal Oxygen Therapy Trial (NOTT) in Chronic Obstructive Pulmonary Disease Patients with Hypoxemia (Ann Intern Med, 1980) [MEDLINE]: n = 203 followed for ≥12 mos (mean: 19.3 mos)
- In Hypoxemic Chronic Obstructive Pulmonary Disease, Continuous Oxygen Therapy (Used at Least 18 hrs Per Day) Had Lower Mortality than Nocturnal Oxygen Therapy: nocturnal oxygen therapy group had 1.94x higher mortality than the continuous oxygen therapy group
- Benefit was Most Significant in Patients with Chronic Hypercapnia
- NOTT and MRC Trials Only Included Patients with More Severe Hypoxemia (pO2 ≤60 mm Hg), as Opposed to Other Trials Which Did Not Demonstrate a Benefit of Long-Term Oxygen Therapy, Which Also Included Less Severely Hypoxemic Patients (pO2 <69 mm Hg) (Am J Respir Crit Care Med, 2006) [MEDLINE]: indicates that COPD patients with less severe hypoxemia may not benefit from long-term oxygen therapy
- In Hypoxemic Chronic Obstructive Pulmonary Disease, Continuous Oxygen Therapy (Used at Least 18 hrs Per Day) Had Lower Mortality than Nocturnal Oxygen Therapy: nocturnal oxygen therapy group had 1.94x higher mortality than the continuous oxygen therapy group
- Medical Research Council (MRC) Trial of Oxygen Therapy in Chronic Obstructive Pulmonary Disease Patients with Severe Hypoxemia, Hypercapnia, and a History of Heart Failure (Lancet, 1981) [MEDLINE]: n = 87
- Oxygen Therapy (Used at Least 15 hrs Per Day) Improved the Mortality Rate
- NOTT and MRC Trials Only Included Patients with More Severe Hypoxemia (pO2 ≤60 mm Hg), as Opposed to Other Trials Which Did Not Demonstrate a Benefit of Long-Term Oxygen Therapy, Which Also Included Less Severely Hypoxemic Patients (pO2 <69 mm Hg) (Am J Respir Crit Care Med, 2006) [MEDLINE]: indicates that COPD patients with less severe hypoxemia may not benefit from long-term oxygen therapy
- Home oxygen for moderate hypoxaemia in chronic obstructive pulmonary disease: a systematic review and meta-analysis. Lancet Respir Med. 2022 Jul 8;S2213-2600(22)00179-5. doi: 10.1016/S2213-2600(22)00179-5 [MEDLINE]
- Background: Long-term oxygen therapy (LTOT) improves survival in patients with chronic obstructive pulmonary disease (COPD) and severe hypoxaemia. However, the best method of management of moderate hypoxaemia not qualifying for LTOT (including isolated nocturnal desaturation) is uncertain. We examined the effect of home oxygen (either LTOT or nocturnal oxygen therapy) on overall survival in patients with COPD and moderate hypoxaemia
- Methods: In this systematic review and meta-analysis, we searched MEDLINE, Embase, the Cochrane Central Register of Controlled Trials, CINHAL, and Web of Science from database inception to Jan 13, 2022, for parallel-group randomised trials of long-term or nocturnal oxygen in patients with COPD and moderate daytime hypoxaemia or isolated nocturnal desaturation, or both. Control groups received usual care or ambient air through sham concentrators (placebo) throughout the study period. The primary outcome of interest was 3-year mortality. Crossover trials and trials of oxygen in severe hypoxaemia were excluded. Two reviewers applied inclusion and exclusion criteria to titles and abstracts and screened the full-text articles and reference lists of relevant studies. Aggregate data were extracted manually in duplicate using structured data collection forms. Methodological quality was assessed using the Cochrane Risk of Bias tool. Random-effects meta-analysis was used to pool individual studies. We considered the minimal clinically important difference for home oxygen to be a relative risk reduction in mortality at 3-year follow-up of 30-40%. The meta-analysis is registered on PROSPERO, CRD42021225372
- Findings: We identified 2192 studies and screened 1447 after removal of duplicates, of which 161 were subjected to full-text screening, and six were identified as being eligible for inclusion. These six randomised trials were published between 1992 and 2020 and the quality of evidence was high. In the primary meta-analysis (five trials; 1002 patients), we found the effect of home oxygen in reducing 3-year mortality to be small or absent (relative risk 0·91 [95% CI 0·72-1·16]; τ2 = 0·00), hence the lower limit of the 95% CI did not meet the prespecified minimal clinically important difference
- Interpretation: The results of our meta-analysis suggest that home oxygen probably makes little or no difference to 3-year mortality in patients with COPD and moderate hypoxaemia. The data do not support the widespread use of home oxygen in this patient population.
- Breathlessness and exercise performance to predict mortality in long-term oxygen therapy – The population-based DISCOVERY study. Respir Med. 2023 Jun 5;216:107306. doi: 10.1016/j.rmed.2023.107306 [MEDLINE]
- Background: Patients with chronic respiratory failure treated with long-term oxygen therapy (LTOT) often have severe breathlessness, impaired exercise performance, and high but variable mortality that is difficult to predict. We aimed to evaluate breathlessness and exercise performance upon starting LTOT as predictors of overall and short-term mortality
- Methods: This was a longitudinal, population-based study of patients who initiated LTOT between 2015 and 2018 in Sweden. Breathlessness was measured using the Dyspnea Exertion Scale, and exercise performance using the 30s-Sit-To-Stand test. Associations with overall and three-month mortality were analyzed using Cox-regression. Subgroup analyses were performed for patients with chronic obstructive pulmonary disease (COPD) and interstitial lung disease (ILD) respectively. The predictive capacity of models was assessed using a C-statistic
- Results: A total of 441 patients (57.6% female, aged 75.4 ± 8.3 years) were analyzed, of whom 141 (32%) died during a median follow-up of 260 (IQR 75-460) days. Both breathlessness and exercise performance were independently associated with overall mortality in the crude models, but only exercise performance remained independently associated with overall mortality when models were adjusted for other predictors, when short-term mortality was analyzed, or when breathlessness and exercise capacity were analyzed concurrently. The multivariable model including exercise performance but not breathlessness provided a relatively high predictive capacity for overall mortality, C-statistic 0.756 (95% CI 0.702-0.810). Similar results were seen in the COPD and ILD subgroups
- Conclusion: Exercise performance as measured by the 30s-STS may be useful to identify patients with higher mortality on LTOT for optimized management and follow-up.
- Effects of long-term oxygen therapy on acute exacerbation and hospital burden: the national DISCOVERY study. Thorax. 2025 May 20;80(6):378-384. doi: 10.1136/thorax-2023-221063 [MEDLINE]
- Background: Long-term oxygen therapy (LTOT) improves survival in patients with chronic severe resting hypoxaemia, but effects on hospitalisation are unknown. This study evaluated the potential impact of starting LTOT on acute exacerbation and hospital burden in patients with chronic obstructive pulmonary disease (COPD), interstitial lung disease (ILD) and pulmonary hypertension (PH)
- Methods: Longitudinal analysis of consecutive patients in the population-based Swedish DISCOVERY cohort who started LTOT between 2000 and 2018 with a follow-up duration≥3 months. Total and hospitalised acute exacerbations of the underlying disease, all-cause hospitalisations, and all-cause outpatient visits were annualised and compared between the year before and after LTOT initiation for each disease cohort, and by hypercapnic status in patients with COPD
- Results: Patients with COPD (n=10 134) had significant reduction in annualised rates of total and hospitalised acute exacerbations, as well as all-cause hospitalisations, following LTOT initiation, with increment in those with ILD (n=2507) and PH (n=850). All-cause outpatient visits increased across all cohorts following LTOT initiation. Similar findings were observed in patients with hypercapnic and non-hypercapnic COPD. Sensitivity analyses of patients with 12 months of follow-up showed reduced acute exacerbations and all-cause hospitalisations in the ILD and PH cohorts
- Conclusion: LTOT is associated with reduced rates of both total and hospitalised acute exacerbations and all-cause hospitalisations in patients with COPD, as well as patients with ILD and PH with 12 months of follow-up. There is increased all-cause outpatient visits in all disease groups following LTOT initiation
Recommendations (Canadian Thoracic Society Guidelines for the Management of Dyspnea in COPD) (Can Respir J, 2011) [MEDLINE]
- Continuous Oxygen Therapy for Hypoxemic Patients Decreases the Mortality Rate and May Decrease Dyspnea in Advanced COPD (Grade 2B Recommendation) (see Oxygen)
- No Evidence to Support the Routine Use of Supplemental Oxygen to Decrease Dyspnea in Normoxemic Patients with Advanced COPD
- There is Little Benefit from Supplemental Oxygen on Quality of Life in Patients with Advanced COPD
Medication Compliance
- Adherence and persistence to once-daily single-inhaler versus multiple-inhaler triple therapy among patients with chronic obstructive pulmonary disease in the USA: a real-world study. Respiratory Medicine. Published online March 18, 2022. doi:10.1016/j.rmed.2022.106807 [MEDLINE]
- Background: Triple therapy comprising an inhaled corticosteroid, long-acting muscarinic antagonist, and long-acting β2 agonist (ICS/LAMA/LABA) is recommended for chronic obstructive pulmonary disease (COPD) patients at risk of exacerbation. Multiple-inhaler triple therapy (MITT) is associated with poor adherence and persistence; however, these outcomes have not been evaluated for single-inhaler fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI)
- Methods: This retrospective analysis of the IQVIA PharMetrics Plus claims database identified patients with COPD initiating triple therapy between 18 September 2017 and 30 June 2019. The first date of single-inhaler FF/UMEC/VI dispensing, or first day of overlapping ICS, LAMA, and LABA medications for MITT users, defined the index date. Patients were ≥40 years, had ≥12 months of continuous insurance coverage pre-index (baseline) and ≥6 months’ coverage post-index; those with MITT during baseline were excluded. Inverse probability weighting was used to balance baseline characteristics. Adherence was assessed using proportion of days covered (PDC) and was evaluated using linear and log-binomial models. Persistence (non-persistence identified as >30-day gap between fills) was evaluated using Cox models
- Results: 9942 patients (FF/UMEC/VI: 2782; MITT: 7160) were included. Adherence was significantly higher for FF/UMEC/VI versus MITT users (mean PDC, 0.66 vs. 0.48; p < 0.001), and FF/UMEC/VI users were twice as likely to be adherent (PDC ≥0.8) than MITT users (46.5% vs. 22.3%; risk ratio [95% CI]: 2.08 [1.85-2.30]; p < 0.001). After 12 months, significantly more FF/UMEC/VI users persisted on therapy than MITT users (35.7% vs. 13.9%; hazard ratio [95% CI]: 1.91 [1.81-2.01]; p < 0.001)
- Conclusions: COPD patients initiating single-inhaler FF/UMEC/VI had significantly improved adherence and persistence compared with MITT.
Dual Therapy vs Triple Therapy
- xxxx
- Clinical and economic outcomes in patients with chronic obstructive pulmonary disease initiating maintenance therapy with tiotropium bromide/olodaterol or fluticasone furoate/umeclidinium/vilanterol. J Manag Care Spec Pharm. 2023 May 3;1-16. doi: 10.18553/jmcp.2023.22373 [MEDLINE]
- BACKGROUND
- Clinical practice guidelines recommend dual long-acting muscarinic antagonists (LAMAs)/long-acting β2agonists (LABAs) as maintenance therapy in patients with chronic obstructive pulmonary disease (COPD) and dyspnea or exercise intolerance
- Escalation to triple therapy (TT) (LAMA/LABA/inhaled corticosteroid) is conditionally recommended for patients with continued exacerbations on dual LAMA/ LABA therapy. Despite this guidance, TT use is widespread across COPD severities, which could impact clinical and economic outcomes
- OBJECTIVE
- To compare COPD exacerbations, pneumonia events, and disease-related and all-cause health care resource utilization and costs (in 2020 US dollars) in patients initiating fixed-dose combinations of either LAMA/ LABA (tiotropium/olodaterol [TIO + OLO]) or TT (fluticasone furoate/umeclidinium/vilanterol [FF + UMEC + VI])
- METHODS
- This retrospective observational study of administrative claims included patients with COPD aged 40 years or older initiating TIO + OLO or FF + UMEC + VI from June 2015 to November 2019. TIO + OLO and FF + UMEC + VI cohorts in the overall and maintenance-naive populations were 1:1 propensity score matched on baseline demographics, comorbidities, COPD medications, health care resource utilization, and costs
- Multivariable regression compared clinical and economic outcomes up to 12 months in FF + UMEC + VI vs TIO + OLO postmatched cohorts
- RESULTS
- After matching, there were 5,658 and 3,025 pairs in the overall and maintenance-naive populations, respectively. In the overall population, the risk of any (moderate or severe) exacerbation was 7% lower in FF + UMEC + VI vs TIO + OLO initiators (adjusted hazard ratio [aHR] = 0.93; 95% CI = 0.86-1.0; P = 0.047)
- There was no difference in the adjusted risk of any exacerbation in the maintenance-naive population (aHR = 0.99; 95% CI = 0.88-1.10). Pneumonia risk was not statistically different between cohorts in the overall (aHR = 1.12; 95% CI = 0.98-1.27) and maintenance-naive (aHR = 1.13; 95% CI = 0.95-1.36) populations
- COPD- and/or pneumonia-related adjusted total annualized costs (95% CI) were significantly greater for FF + UMEC + VI vs TIO + OLO in the overall ($17,633 [16,661-18,604] vs $14,558 [13,709-15,407]; P < 0.001; differences [% of relative increase] = $3,075 [21.1%]) and maintenancenaive ($19,032 [17,466-20,598] vs $15,004 [13,786-16,223]; P < 0.001; $4,028 [26.8%]) populations, with significantly higher pharmacy costs with FF + UMEC + VI (overall: $6,567 [6,503-6,632] vs $4,729 [4,676-4,783]; P < 0.001; $1,838 [38.9%]; maintenance-naive: $6,642 [6,560-6,724] vs $4,750 [4,676-4,825]; P < 0.001; $1,892 [39.8%])
- CONCLUSIONS
- A lower risk of exacerbation was observed with FF + UMEC + VI vs TIO + OLO in the overall population but not among the maintenance-naive population
- Patients with COPD initiating TIO + OLO had lower annualized costs than FF + UMEC + VI initiators in the overall and maintenance-naive populations
- Thus, in the maintenance-naive population, initiation with dual LAMA/LABA therapy per practice guidelines can improve real-world economic outcomes
- BACKGROUND
Short-Acting β2-Adrenergic Receptor Agonists (SABA) (see β2-Adrenergic Receptor Agonists)
- Agents
- Albuterol (Salbutamol, Ventolin) (see Albuterol)
- Levalbuterol (Xopenex) (see Levalbuterol)
- Pirbuterol (Maxair) (see Pirbuterol)
- Recommendation (American College of Chest Physicians/Canadian Thoracic Society Guidelines on the Prevention of COPD Exacerbations, 2015) (Chest, 2015) [MEDLINE]
- In Moderate-Severe COPD, Short-Acting Muscarinic Antagonist Monotherapy (as Compared to Short-Acting β2-Agonist Monotherapy) is Recommended to Prevent Mild-Moderate Acute COPD Exacerbations (Grade 2C Recommendation)
- In Moderate-Severe COPD, Short-Acting Muscarinic Antagonist + Short-Acting β2-Agonist Combination Therapy (as Compared to Short-Acting β2-Agonist Monotherapy) is Recommended to Prevent Moderate Acute COPD Exacerbations (Grade 2B Recommendation)
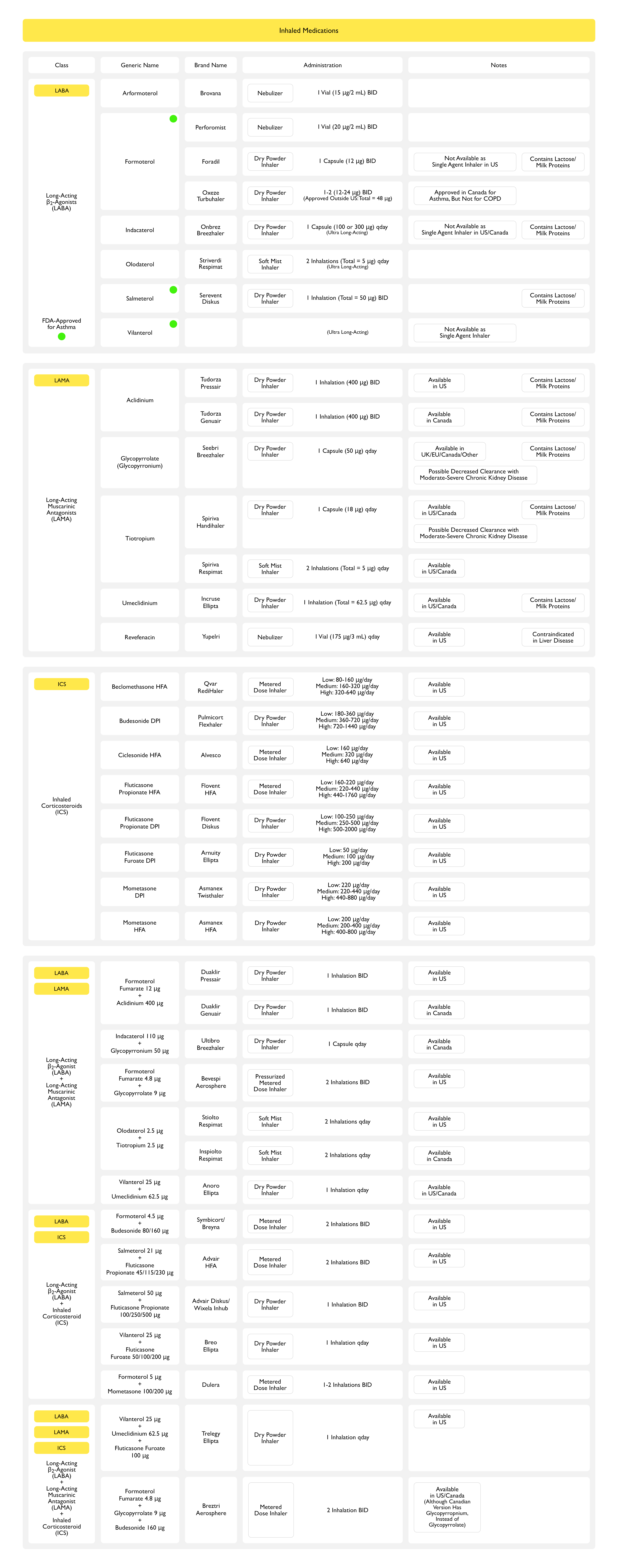
Long-Acting ß2-Adrenergic Agonists (LABA) (see β2-Adrenergic Receptor Agonists)
- Long-Acting ß2-Adrenergic Agonists (LABA) Agents
- Arformoterol (Brovana, Erdotin) (see Arformoterol)
- Bambuterol (Bambec, Oxeol) (see Bambuterol)
- Clenbuterol (Spiropent, Ventipulmin, Dilaterol, Spiropent) (see Clenbuterol)
- Formoterol (Foradil, Oxeze, Oxis, Atock, Atimos, Perforomist) (see Formoterol)
- Formoterol + Budesonide (Symbicort) (see Formoterol + Budesonide)
- Olodaterol (Striverdi Respimat) (see Olodaterol)
- Olodaterol + Tiotropium (Stiolto Respimat) (see Olodaterol-Tiotropium)
- Salmeterol (Serevent) (see Salmeterol)
- Salmeterol + Fluticasone (Advair) (see Salmeterol + Fluticasone)
- Ultra Long-Acting ß2-Adrenergic Agonists (Ultra LABA) Agents
- Indacaterol (Arcapta) (see Indacaterol)
- Olodaterol (Striverdi Respimat) (see Olodaterol)
- Olodaterol + Tiotropium (Stiolto Respimat) (see Olodaterol-Tiotropium)
- Vilanterol(see Vilanterol)
- Vilanterol + Fluticasone (Breo Ellipta) (see Vilanterol + Fluticasone)
- Vilanterol + Umeclidinium (Anoro Ellipta) (see Vilanterol + Umeclidinium)
- Clinical Efficacy
- OPTIMO Trial (Respir Res, 2014) [MEDLINE]
- Withdrawal of Inhaled Corticosteroids in COPD Patients at Low Risk for Exacerbation is Safe, Provided that Patients are Left on Maintenance LABA Therapy
- Systematic Review of LABA Monotherapy vs LAMA Monotherapy vs LABA + LAMA Combination Therapy in COPD (Cochrane Database Syst Rev, 2015) [MEDLINE]
- Combination LABA + LAMA Therapy Resulted in a Small Improvement in Health-Related Quality of Life in COPD, as Compared to LABA Monotherapy or LAMA Monotherapy
- Adding Tiotropium to LABA Decreased the COPD Exacerbation Rate
- Interestingly, Adding LABA to Tiotropium Did Not Decrease the COPD Exacerbation Rate
- Adding LABA to Tiotropium Did Not Decrease the Hospital Admission or Mortality Rate: although there may be insufficient data
- xxxx
- OPTIMO Trial (Respir Res, 2014) [MEDLINE]
- Comparative Effectiveness and Safety of Generic Versus Brand-Name Fluticasone-Salmeterol to Treat Chronic Obstructive Pulmonary Disease. Ann Intern Med. 2023 Aug;176(8):1047-1056. doi: 10.7326/M23-0615 [MEDLINE]
- Background: In 2019, the U.S. Food and Drug Administration (FDA) approved the first generic maintenance inhaler for asthma and chronic obstructive pulmonary disease (COPD). The inhaler, Wixela Inhub (fluticasone-salmeterol; Viatris), is a substitutable version of the dry powder inhaler Advair Diskus (fluticasone-salmeterol; GlaxoSmithKline). When approving complex generic products like inhalers, the FDA applies a special “weight-of-evidence” approach. In this case, manufacturers were required to perform a randomized controlled trial in patients with asthma but not COPD, although the product received approval for both indications
- Objective: To compare the effectiveness and safety of generic (Wixela Inhub) and brand-name (Advair Diskus) fluticasone-salmeterol among patients with COPD treated in routine care
- Design: A 1:1 propensity score-matched cohort study
- Setting: A large, longitudinal health care database
- Patients: Adults older than 40 years with a diagnosis of COPD
- Measurements: Incidence of first moderate or severe COPD exacerbation (effectiveness outcome) and incidence of first pneumonia hospitalization (safety outcome) in the 365 days after cohort entry
- Results: Among 45 369 patients (27 305 Advair Diskus users and 18 064 Wixela Inhub users), 10 012 matched pairs were identified for the primary analysis. Compared with Advair Diskus use, Wixela Inhub use was associated with a nearly identical incidence of first moderate or severe COPD exacerbation (hazard ratio [HR], 0.97 [95% CI, 0.90 to 1.04]) and first pneumonia hospitalization (HR, 0.99 [CI, 0.86 to 1.15])
- Limitations: Follow-up times were short, reflecting real-world clinical practice. The possibility of residual confounding cannot be completely excluded
- Conclusion: Use of generic and brand-name fluticasone-salmeterol was associated with similar outcomes among patients with COPD treated in routine practice.
- Recommendation (American College of Chest Physicians/Canadian Thoracic Society Guidelines on the Prevention of COPD Exacerbations, 2015) (Chest, 2015) [MEDLINE]
- In Moderate-Severe COPD, Long-Acting β2-Agonists (as Compared to Placebo) are Recommended to Prevent Moderate-Severe Acute COPD Exacerbations (Grade 1B (Grade 1B Recommendation)
- In Moderate-Severe COPD, Long-Acting Muscarinic Antagonists (as Compared to Long-Acting β2-Agonists) are Recommended to Prevent Moderate-Severe Acute COPD Exacerbations (Grade 1C Recommendation)
- In Moderate-Severe COPD, Long-Acting β2-Agonist Monotherapy (as Compared to Short-Acting Muscarinic Antagonist Monotherapy) is Recommended to Prevent Acute COPD Exacerbations (Grade 2C Recommendation)
- In Moderate-Severe COPD, Short-Acting Muscarinic Antagonist + Long-Acting β2-Agonist Combination Therapy (as Compared to Long-Acting β2-Agonist Monotherapy) is Recommended to Prevent Mild-Moderate Acute COPD Exacerbations (Grade 2C Recommendation)
- In Stable Moderate-Very Severe COPD, Inhaled Corticosteroid + Long-Acting β2-Agonist Combination Therapy (as Compared to Placebo) is Recommended to Prevent Acute COPD Exacerbations (Grade 1B Recommendation)
- In Stable Moderate-Very Severe COPD, Inhaled Corticosteroid + Long-Acting β2-Agonist Combination Therapy (as Compared to Long-Acting β2-Agonist Monotherapy) is Recommended to Prevent Acute COPD Exacerbations (Grade 1C Recommendation)
- In Stable Moderate-Very Severe COPD, Inhaled Corticosteroid + Long-Acting β2-Agonist Combination Therapy (as Compared to Inhaled Corticosteroid Monotherapy) is Recommended to Prevent Acute COPD Exacerbations (Grade 1B Recommendation)
- In Stable COPD, Long-Acting Muscarinic Antagonist + Long-Acting β2-Agonist Combination Therapy or Long-Acting Muscarinic Antagonist Monotherapy are Both Recommended to Prevent Acute COPD Exacerbations (Grade 1C Recommendation): both are equally effective
- In Stable COPD, Inhaled Corticosteroid + Long-Acting β2-Agonist Combination Therapy or Long-Acting Muscarinic Antagonist Monotherapy are Both Recommended to Prevent Acute COPD Exacerbations (Grade 1C Recommendation): both are equally effective
- In Stable COPD, Inhaled Corticosteroid + Long-Acting Muscarinic Antagonist + Long-Acting β2-Agonist Triple Therapy or Long-Acting Muscarinic Antagonist Monotherapy are Both Recommended to Prevent Acute COPD Exacerbations (Grade 2C Recommendation): both are equally effective
Anticholinergic (Muscarinic Antagonist) Agents (see Muscarinic Antagonists)
- Short-Acting Muscarinic Antagonists
- Ipratropium Bromide (Atrovent) (see Ipratropium Bromide)
- Long-Acting Muscarinic Antagonists
- Aclidinium (Tudorza Pressair) (see Aclidinium)
- Glycopyrronium Bromide (see Glycopyrronium Bromide)
- Tiotropium (Spiriva) (see Tiotropium) [MEDLINE]: antagonist at airway M2 and M3 muscarinic receptors
- Umeclidinium (Incruse) (see Umeclidinium)
- Vilanterol + Umeclidinium (Anoro Ellipta) (see Vilanterol-Umeclidinium)
- Clinical Efficacy
- Systematic Review of LABA Monotherapy vs LAMA Monotherapy vs LABA + LAMA Combination Therapy in COPD (Cochrane Database Syst Rev, 2015) [MEDLINE]
- Combination LABA + LAMA Therapy Resulted in a Small Improvement in Health-Related Quality of Life in COPD, as Compared to LABA Monotherapy or LAMA Monotherapy
- Adding Tiotropium to LABA Decreased the COPD Exacerbation Rate
- Interestingly, Adding LABA to Tiotropium Did Not Decrease the COPD Exacerbation Rate
- Adding LABA to Tiotropium Did Not Decrease the Hospital Admission or Mortality Rate: although there may be insufficient data
- xxx
- Randomized ETHOS Trial of Budesonide/Glycopyrrolate/Formoterol vs Glycopyrrolate/Formoterol or Budesonide/Formoterol (NEJM, 2020) [MEDLINE]
- Triple Therapy with Twice Daily Budesonide (at Either the 160 μg or 320 μg dose), Glycopyrrolate, and Formoterol Resulted in a Lower Rate of Moderate or Severe COPD Exacerbations than Glycopyrrolate/Formoterol or Budesonide/Formoterol
- Systematic Review of LABA Monotherapy vs LAMA Monotherapy vs LABA + LAMA Combination Therapy in COPD (Cochrane Database Syst Rev, 2015) [MEDLINE]
- Recommendation (American College of Chest Physicians/Canadian Thoracic Society Guidelines on the Prevention of COPD Exacerbations, 2015) (Chest, 2015) [MEDLINE]
- In Moderate-Severe COPD, Long-Acting Muscarinic Antagonists (as Compared to Placebo) are Recommended to Prevent Moderate-Severe Acute COPD Exacerbations (Grade 1A Recommendation)
- In Moderate-Severe COPD, Long-Acting Muscarinic Antagonists (as Compared to Long-Acting β2-Agonists) are Recommended to Prevent Moderate-Severe Acute COPD Exacerbations (Grade 1C Recommendation)
- In Moderate-Severe COPD, Short-Acting Muscarinic Antagonist Monotherapy (as Compared to Short-Acting β2-Agonist Monotherapy) is Recommended to Prevent Mild-Moderate Acute COPD Exacerbations (Grade 2C Recommendation)
- In Moderate-Severe COPD, Short-Acting Muscarinic Antagonist + Short-Acting β2-Agonist Combination Therapy (as Compared to Short-Acting β2-Agonist Monotherapy) is Recommended to Prevent Moderate Acute COPD Exacerbations (Grade 2B Recommendation)
- In Moderate-Severe COPD, Long-Acting β2-Agonist Monotherapy (as Compared to Short-Acting Muscarinic Antagonist Monotherapy) is Recommended to Prevent Acute COPD Exacerbations (Grade 2C Recommendation)
- In Moderate-Severe COPD, Long-Acting Muscarinic Antagonist Monotherapy (as Compared to Short-Acting Muscarinic Antagonist Monotherapy) is Recommended to Prevent Moderate-Severe Acute COPD Exacerbations (Grade 1A Recommendation)
- In Moderate-Severe COPD, Short-Acting Muscarinic Antagonist + Long-Acting β2-Agonist Combination Therapy (as Compared to Long-Acting β2-Agonist Monotherapy) is Recommended to Prevent Mild-Moderate Acute COPD Exacerbations (Grade 2C Recommendation)
- In Stable COPD, Long-Acting Muscarinic Antagonist + Long-Acting β2-Agonist Combination Therapy or Long-Acting Muscarinic Antagonist Monotherapy are Both Recommended to Prevent Acute COPD Exacerbations (Grade 1C Recommendation): both are equally effective
- In Stable COPD, Inhaled Corticosteroid + Long-Acting β2-Agonist Combination Therapy or Long-Acting Muscarinic Antagonist Monotherapy are Both Recommended to Prevent Acute COPD Exacerbations (Grade 1C Recommendation): both are equally effective
- In Stable COPD, Inhaled Corticosteroid + Long-Acting Muscarinic Antagonist + Long-Acting β2-Agonist Triple Therapy or Long-Acting Muscarinic Antagonist Monotherapy are Both Recommended to Prevent Acute COPD Exacerbations (Grade 2C Recommendation): both are equally effective
HMG-CoA Reductase Inhibitors (Statins) (see HMG-CoA Reductase Inhibitors)
- Clinical Efficacy
- Prospective Randomized Placebo-Controlled Trial of Simvastatin in the Prevention of COPD Exacerbations (STATCOPE) Trial (NEJM, 2014) [MEDLINE]: randomized, controlled trial of simvastatin (40 mg daily) versus placebo -> primary outcome: annual exacerbation rate
- Simvastatin Had No Effect on the COPD Exacerbation Rate, Time to First Exacerbation, or Cardiac Events
- Danish Study of Impact of Statins on the COPD Exacerbation Rate (Thorax, 2015) [MEDLINE]: Copenhagen General Population Study (2003-2008)
- Statins Decreased the COPD Exacerbation Rate in General Population, Although This was Not Demonstrated in the Most Severe COPD Patients without Cardiovascular Comorbidity: suggesting that statins may decrease the risk of exacerbations only in patients with coexisting cardiovascular disease
- Prospective Randomized Placebo-Controlled Trial of Simvastatin in the Prevention of COPD Exacerbations (STATCOPE) Trial (NEJM, 2014) [MEDLINE]: randomized, controlled trial of simvastatin (40 mg daily) versus placebo -> primary outcome: annual exacerbation rate
- Recommendation (American College of Chest Physicians/Canadian Thoracic Society Guidelines on the Prevention of COPD Exacerbations, 2015) (Chest, 2015) [MEDLINE]
- In Moderate-Severe COPD at Risk for Exacerbation, Statins are Not Recommended to Decrease the COPD Exacerbation Rate (Grade 1B Recommendation)
Inhaled Corticosteroids (see Corticosteroids)
- Clinical Efficacy-Exacerbation Rate in COPD
- ISOLDE Trial in Moderate-Severe COPD (Br Med J, 2000) [MEDLINE]
- Fluticasone Decreased the COPD Exacerbation rate, Increased FEV1 Slightly, and Resulted in a Slower Decline in Health Status
- Torch Trial Data. Trial of Salmeterol and Fluticasone in COPD (NEJM, 2007) [MEDLINE]
- Salmeterol and Fluticasone Did Not Impact the Mortality Rate, But Decreased the COPD Exacerbation Tate
- Salmeterol and Fluticasone Increased the Pneumonia Rate in COPD
- Systematic Review of Combination Corticosteroid + LABA Inhaler in COPD (2013) [MEDLINE]
- Combination Corticosteroid + LABA Inhaler Decreased the Exacerbation Rate and Decreased All-Cause Mortality (Although Latter Finding was Due Primarily to the TORCH Trial)
- Combination Corticosteroid + LABA Inhaler Increased the Pneumonia Rate (But Without an Increase in Exacerbations, Hospitalizations, or Deaths)
- Inadequate data to determine superiority of one combination over another
- Randomized ETHOS Trial of Budesonide/Glycopyrrolate/Formoterol vs Glycopyrrolate/Formoterol or Budesonide/Formoterol (NEJM, 2020) [MEDLINE]
- Triple Therapy with Twice Daily Budesonide (at Either the 160 μg or 320 μg dose), Glycopyrrolate, and Formoterol Resulted in a Lower Rate of Moderate or Severe COPD Exacerbations than Glycopyrrolate/Formoterol or Budesonide/Formoterol
- ISOLDE Trial in Moderate-Severe COPD (Br Med J, 2000) [MEDLINE]
- Clinical Efficacy-Mortality Rate Large Meta-Analysis of Inhaled Corticosteroid Maintenance Therapy in COPD (Chest, 2022) [MEDLINE]: n = 103,034 (from 60 randomized controlled trials) Inhaled therapy containing ICSs (Peto OR, 0.90; 95% CI, 0.84-0.97), especially triple therapy (Peto OR, 0.73; 95% CI, 0.59-0.91), was associated with a reduction in the cause-death risk among COPD patients when compared to inhaled therapy without ICSs. Subgroup analyses revealed that treatment duration >6 mos (Peto OR, 0.90; 95% CI, 0.83-0.97), medium-dose (Peto OR, 0.71; 95% CI, 0.56-0.91)/low-dose ICSs (Peto OR, 0.88; 95% CI, 0.79-0.97), and budesonide (Peto OR, 0.75; 95% CI, 0.59-0.94) were involved in this association Predictors of This Association Eosinophil Count ≥200/μL or Eosinophil Percentage ≥2%: eosinophil counts ≥200/μL (Peto OR, 0.58; 95% CI, 0.36-0.95) was the strongest predictor Documented History of ≥2 Moderate-Severe Exacerbations in the Previous Year GOLD Stage III-IV Age <65 y/o BMI ≥25
- Inhaled Corticosteroids Versus Placebo for Stable Chronic Obstructive Pulmonary Disease: A Review. Clin Exp Allergy. 2024 Jun 12. doi: 10.1111/cea.14521 [MEDLINE]
- Cochrane systematic review included 36 placebo-controlled trials, involving 23,139 participants and aimed to evaluate the ben- efits and harms of using ICS as monotherapy when compared to placebo in people with stable COPD
- This systematic review updates the evidence base for ICS monotherapy with newly published trials to aid the ongoing assessment of their role for people with COPD
- Use of ICS alone for COPD likely results in a reduction in exacerbation rates of clinical relevance, probably results in a reduction in the rate of decline of FEV1 of uncertain clinical relevance and likely results in a small improvement in health-related quality of life not meeting the threshold for a minimally clinically import- ant difference
- These potential benefits should be weighed up against adverse events (likely to increase local oropharyngeal adverse effects and may increase the risk of pneumonia) and probably no reduction in mortality
- Though not recommended as monotherapy, the probable benefits of ICS highlighted in this review support their continued consideration in combination with long-acting bronchodilators
- Future research and evidence syntheses should be focused in that area.
- Clinical Efficacy-Risk of Pneumonia in Chronic Obstructive Pulmonary Disease (COPD)
- TORCH Study (Eur Respir J, 2009) [MEDLINE]
- Inhaled Corticosteroids Increased the Risk of Pneumonia in COPD
- XXXX
- TORCH Study (Eur Respir J, 2009) [MEDLINE]
- Inhaled Corticosteroids in Patients with Chronic Obstructive Pulmonary Disease and Risk of Acquiring Streptococcus pneumoniae Infection. A Multiregional Epidemiological Study. Int J Chron Obstruct Pulmon Dis. 2023 Mar 21;18:373-384. doi: 10.2147/COPD.S386518. eCollection 2023 [MEDLINE]
- Background: Inhaled corticosteroids (ICS) are associated with an increased risk of clinical pneumonia among patients with chronic obstructive pulmonary disease (COPD). It is unknown whether the risk of microbiologically verified pneumonia such as pneumococcal pneumonia is increased in ICS users.
- Methods: The study population consists of all COPD patients followed in outpatient clinics in eastern Denmark during 2010-2017. ICS use was categorized into four categories based on accumulated use. A Cox proportional hazard regression model was used adjusting for age, body mass index, sex, airflow limitation, use of oral corticosteroids, smoking, and year of cohort entry. A propensity score matched analysis was performed for sensitivity analyses.
- Findings: A total of 21,438 patients were included. Five hundred and eighty-two (2.6%) patients acquired a positive lower airway tract sample with S. pneumoniae during follow-up. In the multivariable analysis ICS-use was associated with a dose-dependent risk of S. pneumoniae as follows: low ICS dose: HR 1.11, 95% CI 0.84 to 1.45, p = 0.5; moderate ICS dose: HR 1.47, 95% CI 1.13 to 1.90, p = 0.004; high ICS dose: HR 1.77, 95% CI 1.38 to 2.29, p < 0.0001, compared to no ICS use. Sensitivity analyses confirmed these results.
- Interpretation: Use of ICS in patients with severe COPD was associated with an increased and dose-dependent risk of acquiring S. pneumoniae, but only for moderate and high dose. Caution should be taken when administering high dose of ICS to patients with COPD. Low dose of ICS seemed not to carry this risk.
- The impact of inhaled corticosteroids on the prognosis of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2023 May 2;18:733-743. doi: 10.2147/COPD.S388367. eCollection 2023 [MEDLINE]
- Background: A comprehensive analysis of the effects of inhaled corticosteroids (ICS) on COPD in a real-world setting is required due to safety concerns regarding ICS in COPD. This study aimed to explore the impact of ICS on the prognosis of Asian COPD patients in the real-life world
- Methods: We examined 978 COPD patients registered in the Korean National Health and Nutrition Examination Survey (KNHANES) database and with their data linked to Health Insurance and Review Assessment (HIRA) data. The outcome measures were ascertained by HIRA from January 1, 2009, to December 31, 2012. This study enrolled two arms; ICS users (N = 85, mean age = 66.7 ± 8.9 years) and non-ICS users (N = 893, mean age = 63.7 ± 9.7 years)
- Results: Compared to the non-ICS users, the ICS users had a higher rate of pneumonia, tuberculosis, and acute exacerbations (P<0.05). Hospitalization due to respiratory causes was also higher among ICS users (P<0.05). Multivariate analysis showed that acute exacerbation was independently associated with the development of pneumonia (P<0.05), whereas ICS therapy had a tendency to be associated with pneumonia. Another multivariate analysis demonstrated that old age, FEV1, ICS therapy, and pneumonia were independently associated with the occurrence of acute exacerbation (P<0.05). The concomitant pneumonia (HR = 3.353, P = 0.004) was independently associated with higher mortality (P<0.05)
- Conclusion: Our data demonstrated that the ICS users had a higher rate of pneumonia and tuberculosis and the concomitant pneumonia was independently associated with higher mortality, highlighting the importance of cautious and targeted administration of ICS in COPD
- Inhalation devices and inhaled corticosteroids particle size influence on severe pneumonia in patients with chronic obstructive pulmonary disease: a nationwide cohort study. BMJ Open Respir Res. 2023;10(1):e001814. doi:10.1136/bmjresp-2023-001814 [MEDLINE]
- Background: Inhaled corticosteroids (ICSs) are associated with an increased risk of pneumonia among patients with chronic obstructive pulmonary disease (COPD). The introduction of extrafine particle ICS has aimed to improve the distribution of medicine in the airways by altering deposition within the lungs, potentially affecting efficacy and side effects. It remains unclear if extrafine particle ICS administration alters the risk of pneumonia compared with standard particle size ICS
- Methods: An observational cohort study including all Danish COPD outpatients receiving ICS from 2010 to 2017. The primary outcome was pneumonia hospitalisation in the different ICS particle dosing regimens. The primary analysis was an adjusted Cox proportional hazards model. For sensitivity analysis, a subgroup analysis of patients receiving spray devices was done. Further, we created a propensity score matched cohort, in which we matched for the same covariates as adjusted for in the main analysis
- Results: A total of 35 691 patients were included of whom 1471 received extrafine particle ICS. Among these patients, 4657 were hospitalised due to pneumonia. Patients with COPD receiving extrafine particle ICS had a lower risk of hospitalisation due to pneumonia compared with patients receiving standard particle size ICS in our primary analysis (HR 0.75; 95% CI 0.63 to 0.89; p=0.002), subgroup analysis (HR 0.54; 95% CI 0.45 to 0.65; p<0.0001) and the propensity-matched population (HR 0.72; 95% CI 0.60 to 0.87; p=0.0006)
- Interpretation: The use of extrafine particle ICS administration was associated with a lower risk of pneumonia hospitalisation in patients with COPD compared with those who received standard size treatment.
- Clinical factors linked to the type of respiratory medication in COPD: Results from the COSYCONET cohort. Ther Adv Respir Dis. January-December 2023;17:17534666231208584. doi:10.1177/17534666231208584 [MEDLINE]
- Background: The use of maintenance medication in patients with chronic obstructive pulmonary disease (COPD) in real life is known to deviate from recommendations in guidelines, which are largely based on randomized controlled trials and selected populations
- Objectives: We used the COSYCONET (COPD and Systemic Consequences – Comorbidities Network) cohort to analyze factors linked to the use of COPD drugs under non-interventional circumstances
- Design: COSYCONET is an ongoing, multi-center, non-interventional cohort of patients with COPD
- Methods: Patients with COPD of Global Initiative for Chronic Obstructive Lung Disease (GOLD) grades 0-4 participating in visits 1-5 were included. Data covered the period from 2010 to 2018. Generalized linear models were used to examine the relation of COPD characteristics to different types of respiratory medication
- Results: A total of 1043 patients were included. The duration of observation was 4.5 years. Use of respiratory medication depended on GOLD grades 0-4 and groups A-D. Long-acting muscarinic antagonist therapy increased over time, and was associated with low carbon monoxide (CO) diffusing capacity, while inhaled corticosteroid (ICS) use decreased. Active smoking was associated with less maintenance therapy in general, and female sex with less ICS use. From the eight items of the COPD Assessment Test, only hill and stair climbing were consistently linked to treatment
- Conclusion: Using data from a large, close to real-life observational cohort, we identified factors linked to the use of various types of respiratory COPD medication. Overall, use was consistent with GOLD recommendations. Beyond this, we identified other correlates of medication use that may help us to understand and improve therapy decisions in clinical practice.
- Clinical Efficacy-Risk of Parapneumonic Effusion in Association with Pneumonia (in Both Asthma and COPD)
- Spanish Study of the Effect of Prior Inhaled Corticosteroids (in Both Asthma and COPD) on the Risk of Developing Parapneumonic Effusion in Association with Pneumonia (Am J Respir Crit Care Med, 2013) [MEDLINE]
- Prior Use of Inhaled Corticosteroids Decreased the Risk of Parapneumonic Effusion in Association with Pneumonia
- Prior Use of Inhaled Corticosteroids was Associated with Higher Pleural pH, Higher Pleural Glucose, Lower Pleural Protein, and Lower Pleural LDH
- Spanish Study of the Effect of Prior Inhaled Corticosteroids (in Both Asthma and COPD) on the Risk of Developing Parapneumonic Effusion in Association with Pneumonia (Am J Respir Crit Care Med, 2013) [MEDLINE]
- Clinical Efficacy-Withdrawal of Inhaled Corticosteroids
- OPTIMO Trial (Respir Res, 2014) [MEDLINE]
- Withdrawal of Inhaled Corticosteroids in COPD Patients at Low Risk for Exacerbation is Safe, Provided that Patients are Left on Maintenance LABA Therapy
- WISDOM Trial Examining Withdrawal of Inhaled Corticosteroids in Severe Stable COPD Which is Managed with Tiotropium and Salmeterol (NEJM, 2014) [MEDLINE]
- Withdrawal of Inhaled Corticosteroids (in COPD Patients on Tiotropium and Salmeterol) Had No Effect on Risk of Moderate-Severe Exacerbations or Dyspnea, But Led to a Minimal Decrease in FEV1 (38 mL)
- OPTIMO Trial (Respir Res, 2014) [MEDLINE]
- Recommendation (American College of Chest Physicians/Canadian Thoracic Society Guidelines on the Prevention of COPD Exacerbations, 2015) (Chest, 2015) [MEDLINE]
- In Stable Moderate-Very Severe COPD, Inhaled Corticosteroid + Long-Acting β2-Agonist Combination Therapy (as Compared to Placebo) is Recommended to Prevent Acute COPD Exacerbations (Grade 1B Recommendation)
- In Stable Moderate-Very Severe COPD, Inhaled Corticosteroid + Long-Acting β2-Agonist Combination Therapy (as Compared to Long-Acting β2-Agonist Monotherapy) is Recommended to Prevent Acute COPD Exacerbations (Grade 1C Recommendation)
- In Stable Moderate-Very Severe COPD, Inhaled Corticosteroid + Long-Acting β2-Agonist Combination Therapy (as Compared to Inhaled Corticosteroid Monotherapy) is Recommended to Prevent Acute COPD Exacerbations (Grade 1B Recommendation)
- In Stable COPD, Inhaled Corticosteroid + Long-Acting β2-Agonist Combination Therapy or Long-Acting Muscarinic Antagonist Monotherapy are Both Recommended to Prevent Acute COPD Exacerbations (Grade 1C Recommendation): both are equally effective
- In Stable COPD, Inhaled Corticosteroid + Long-Acting Muscarinic Antagonist + Long-Acting β2-Agonist Triple Therapy or Long-Acting Muscarinic Antagonist Monotherapy are Both Recommended to Prevent Acute COPD Exacerbations (Grade 2C Recommendation): both are equally effective
Systemic Corticosteroids (see Corticosteroids)
- Agents
- Prednisone (see Prednisone)
- Recommendation (American College of Chest Physicians/Canadian Thoracic Society Guidelines on the Prevention of COPD Exacerbations, 2015) (Chest, 2015) [MEDLINE]
- For Patients with Acute COPD Exacerbation in Inpatient/Outpatient Setting, Systemic Corticosteroids (Orally or Intravenously) are Recommended to Prevent Hospitalization for Subsequent Acute COPD Exacerbation in the the Next 30 Days Following the Initial Exacerbation (Grade 2B Recommendation)
- For Patients with Acute COPD Exacerbation in Inpatient/Outpatient Setting, Systemic Corticosteroids (Orally or Intravenously) are Not Recommended For the Sole Purpose of Preventing Hospitalization for Subsequent Acute COPD Exacerbation Beyond the First 30 Days Following the Initial Exacerbation (Grade 1A Recommendation)
Secretion Clearance
Rationale
- xxxx
Mucolytics
- Agents
- Carbocysteine (see Carbocysteine)
- N-Acetylcysteine: cleaves disulfide bonds which cross-link glycoproteins in mucus -> results in decreased mucus viscosity, facilitating airway mucus clearance
- Clinical Efficacy
- Systematic Review of Mucolytics in Outpatients with Chronic Bronchitis or Chronic Obstructive Pulmonary Disease (Cochrane Database Syst Rev, 2019) [MEDLINE]
- In participants with chronic bronchitis or COPD, we are moderately confident that treatment with mucolytics leads to a small reduction in the likelihood of having an acute exacerbation, in days of disability per month and possibly hospitalisations, but is not associated with an increase in adverse events
- There appears to be limited impact on lung function or health-related quality of life
- Results are too imprecise to be certain whether or not there is an effect on mortality
- Our confidence in the results is reduced by high levels of heterogeneity in many of the outcomes and the fact that effects on exacerbations shown in early trials were larger than those reported by more recent studies
- This may be a result of greater risk of selection or publication bias in earlier trials, thus benefits of treatment may not be as great as was suggested by previous evidence
- Chinese PANTHEON N-Acetylcysteine Trial (Lancet Respir Med, 2014) [MEDLINE]: prospective, randomized, double-blind, placebo-controlled trial of N-Acetylcysteine performed in China, n = 1006 -> primary endpoint was the annual COPD exacerbation rate
- In Moderate-Severe COPD, Long-Term Use of N-Acetylcysteine (600 mg PO BID) Decreased the Number of Exacerbations (Especially in the Moderate Disease Subgroup)
- N-Acetylcysteine Probably Exerts its Effect Via a Mucolytic Action: other potential mechanisms, such as the generation or neutralization of reactive species (with a potential anti-inflammatory effect), are less supported by the available data
- Systematic Review of Mucolytics in Outpatients with Chronic Bronchitis or Chronic Obstructive Pulmonary Disease (Cochrane Database Syst Rev, 2019) [MEDLINE]
- Recommendation (American College of Chest Physicians/Canadian Thoracic Society Guidelines on the Prevention of COPD Exacerbations, 2015) (Chest, 2015) [MEDLINE]
- In Moderate-Severe COPD and a History of ≥2 COPD Exacerbations in the Prior 2 Years, Oral N-Acetylcysteine is Recommended to Decrease the COPD Exacerbation Rate (Grade 2B Recommendation)
- In Stable Outpatient COPD with Continued Acute COPD Exacerbations Despite Maximal Therapy, Oral Carbocysteine Can Be Used to Decrease the COPD Exacerbation Rate (Ungraded Consensus-Based Statement)
- Mucolytics for acute exacerbations of chronic obstructive pulmonary disease: a meta-analysis. Eur Respir Rev. 2023 Jan 25;32(167):220141. doi: 10.1183/16000617.0141-2022. Print 2023 Mar 31 [MEDLINE]
- This meta-analysis explored the safety and effectiveness of mucolytics as an add-on treatment for chronic obstructive pulmonary disease (COPD) exacerbations
- Based on a pre-registered protocol and following Cochrane methods, we systematically searched for relevant randomised or quasi-randomised controlled trials (RCTs)
- We used the Risk of Bias v2 tool for appraising the studies and performed random-effect meta-analyses when appropriate
- We assessed certainty of evidence using GRADE
- This meta-analysis included 24 RCTs involving 2192 patients with COPD exacerbations, entailing at least some concerns of methodological bias. We demonstrated with moderate certainty that mucolytics increase the rate of treatment success (relative risk 1.37, 95% CI 1.08-1.73, n=383), while they also exert benefits on overall symptom scores (standardised mean difference 0.86, 95% CI 0.63-1.09, n=316), presence of cough at follow-up (relative risk 1.93, 95% CI 1.15-3.23) and ease of expectoration (relative risk 2.94, 95% CI 1.68-5.12)
- Furthermore, low or very low certainty evidence suggests mucolytics may also reduce future risk of exacerbations and improve health-related quality of life, but do not impact on breathlessness, length of hospital stay, indication for higher level of care or serious adverse events. Overall, mucolytics could be considered for COPD exacerbation management
- These findings should be validated in further, rigorous RCTs.
- The effect of nebulized N-acetylcysteine on the phlegm of chronic obstructive pulmonary disease: the NEWEST study. BMC Pulm Med. Published online September 2, 2024. doi:10.1186/s12890-024-03243-y [MEDLINE]
- Background: Phlegm is prevalent symptom in patients with chronic obstructive pulmonary disease (COPD). Few studies have investigated the effectiveness of N-acetylcysteine (NAC) nebulizer therapy in COPD patients. We evaluated the effect of nebulized NAC on the improvement of phlegm symptom in COPD patients
- Methods: This was a 12-week, prospective, single-arm, open-label, phase IV multi-center trial (NCT05102305, Registration Date: 20-October-2021). We enrolled patients aged ≥ 40 years with post bronchodilator forced expiratory volume in one second/forced vital capacity (FEV1/FVC) < 0.7 and COPD assessment test (CAT) phlegm score ≥ 2; the patients were current or ex-smoker with smoking pack-years ≥ 10. The primary endpoint was to determine the change in CAT phlegm score at 12 weeks compared to the baseline. Patients were assessed at baseline, 4, 8, and 12 weeks of treatment using the CAT score
- Results: In total, 100 COPD patients were enrolled from 10 hospitals. The mean age of the patients was 71.42 ± 8.20 years, with 19.78% being current-smokers and 80.22% being ex-smokers. The mean smoking pack-years was 40.32 ± 35.18. The mean FVC, FEV1, and FEV1/FVC were 3.94 L (75.44%), 2.22 L (58.50%), and 0.53, respectively. The CAT phlegm score at baseline was 3.47 ± 1.06, whereas after 12 weeks of nebulized NAC it significantly decreased to 2.62 ± 1.30 (p < 0.01). More than half (53.5%) of the patients expressed satisfaction with the effects of nebulized NAC therapy. Adverse events occurred in 8 (8.0%) patients. Notably, no serious adverse drug reactions were reported
- Conclusion: In this study, we have established the effectiveness and safety of nebulized NAC over 12 weeks
Oscillatory Positive Expiratory Pressure Therapy (see Oscillatory Positive Expiratory Pressure)
- Brands
- Acapella
- Aerobika
- Clinical Efficacy
- O-COPD Randomized Trial of Positive Expiratory Pressure Therapy in Chronic Obstructive Pulmonary Disease (Thorax, 2022) [MEDLINE]
- n = 122 (61/61 Oscillatory Positive Expiratory Pressure Therapy/Control) were recruited (103 completed the study: 55/48 Oscillatory Positive Expiratory Pressure Therapy/Control)
- 40% female
- 17% smokers
- FEV1 38 (25-56)% Predicted
- Age 62±10 y/o
- Use of Oscillatory Positive Expiratory Pressure was Associated with Improvement in Leicester Cough Questionnaire, as Compared to Control; Median 1.03 (95% CI: 0.71 to 2.10; p = 0.03), Functional Assessment of Chronic Illness Therapy Score 4.68 (95% CI: 1.34 to 8.02; p<0.001), and EuroQol-5 Dimensions Score 4.00 (95% CI: 0.49 to 19.75; p=0.04)
- There was Also an Improvement in Cough Frequency -60 (-43 to -95) Coughs/24 hours (p<0.001), But No Statistically Significant Effect on Sleep Disturbance was Identified
- Regular Use of an Acapella Device Improves Symptoms and Quality of life in COPD Patients Who Produce Sputum Daily or Most Days
- n = 122 (61/61 Oscillatory Positive Expiratory Pressure Therapy/Control) were recruited (103 completed the study: 55/48 Oscillatory Positive Expiratory Pressure Therapy/Control)
- O-COPD Randomized Trial of Positive Expiratory Pressure Therapy in Chronic Obstructive Pulmonary Disease (Thorax, 2022) [MEDLINE]
Azithromycin (Zithromax) (see Azithromycin)
- Pharmacology: macrolide antibiotic (see Macrolides)
- Administration: 250 mg PO qday x 1 year
- Adverse Effects
- Hearing Loss (see Hearing Loss): with azithromycin x 1 year, hearing decrements were more common in the azithromycin group than in the placebo group (25% vs. 20%, p = 0.04) (NEJM, 2011) [MEDLINE]
- Drug-Induced Pulmonary Eosinophilia (see Drug-Induced Pulmonary Eosinophilia)
- Increased General Cardiovascular Risk
- Increased Risk of Acute Myocardial Infarction (MI) (see Coronary Artery Disease)
- Risk of Q-T Prolongation with Definite Association with Torsade (see Torsade)
- Clinical Efficacy
- COPD Clinical Research Network Daily Azithromycin Trial (NEJM, 2011) [MEDLINE]: randomized, placebo-controlled trial (n = 1577) with daily azithromycin (250 mg PO) x 1 year
- Exclusion Criteria: asthma, a resting HR >100 beats, prolonged QTc >450 msec, use of medications that prolong the QTc or are associated with torsades (with the exception of amiodarone), and hearing impairment
- Azithromycin Decreased the COPD Exacerbation Rate (1.48 vs 1.83 Per Year)
- Azithromycin Improved QOL
- Azithromycin Decreased Colonization with Selected Respiratory Pathogens (But Increased Colonization with Macrolide-Resistant Organisms)
- Azithromycin Resulted in a Small Increase in Hearing Decrements (25% vs. 20%, p = 0.04)
- No Clear Impact on Microbial Resistance Patterns
- Predictors of COPD Exacerbation Reduction in Response to Daily Azithromycin Therapy (Am J Resp Crit Care Med, 2014) [MEDLINE]
- Azithromycin is Most Effective in Preventing COPD Exacerbations in Patients Requiring Both Antibiotic and Corticosteroid Treatment
- Variables Which Did Not Affect Azithromycin Efficacy: sex, history of chronic bronchitis, oxygen use, or concomitant COPD therapy
- Variables Associated with Increased Azithromycin Efficacy: older age, milder Global Initiative for Chronic Obstructive Lung Disease stage
- Variables Associated with Decreased Azithromycin Efficacy: current tobacco abuse
- COPD Clinical Research Network Daily Azithromycin Trial (NEJM, 2011) [MEDLINE]: randomized, placebo-controlled trial (n = 1577) with daily azithromycin (250 mg PO) x 1 year
- Recommendation (American College of Chest Physicians/Canadian Thoracic Society Guidelines on the Prevention of COPD Exacerbations, 2015) (Chest, 2015) [MEDLINE]
- In Moderate-Severe COPD with History of ≥1 Moderate-Severe COPD Exacerbations in Prior Year Despite Optimal Maintenance Inhaler Therapy, Long-Term Macrolide is Recommended to Decrease the COPD Exacerbation Rate (Grade 2A Recommendation)
Dupilumab (Dupixent) (see Dupilumab)
Clinical Efficacy
- XXXXX
- BOREAS Trial. Dupilumab for COPD with Type 2 Inflammation Indicated by Eosinophil Counts. N Engl J Med. 2023 Jul 20;389(3):205-214. doi: 10.1056/NEJMoa2303951 [MEDLINE]
- Background: In some patients with chronic obstructive pulmonary disease (COPD), type 2 inflammation may increase exacerbation risk and may be indicated by elevated blood eosinophil counts. Dupilumab, a fully human monoclonal antibody, blocks the shared receptor component for interleukin-4 and interleukin-13, key drivers of type 2 inflammation
- Methods: In a phase 3, double-blind, randomized trial, we assigned patients with COPD who had a blood eosinophil count of at least 300 per microliter and an elevated exacerbation risk despite the use of standard triple therapy to receive dupilumab (300 mg) or placebo subcutaneously once every 2 weeks. The primary end point was the annualized rate of moderate or severe exacerbations of COPD. Key secondary and other end points that were corrected for multiplicity were the change in the prebronchodilator forced expiratory volume in 1 second (FEV1) and in the scores on the St. George’s Respiratory Questionnaire (SGRQ; range, 0 to 100, with lower scores indicating a better quality of life) and the Evaluating Respiratory Symptoms in COPD (E-RS-COPD; range, 0 to 40, with lower scores indicating less severe symptoms)
- Results: A total of 939 patients underwent randomization: 468 to the dupilumab group and 471 to the placebo group. The annualized rate of moderate or severe exacerbations was 0.78 (95% confidence interval [CI], 0.64 to 0.93) with dupilumab and 1.10 (95% CI, 0.93 to 1.30) with placebo (rate ratio, 0.70; 95% CI, 0.58 to 0.86; P<0.001). The prebronchodilator FEV1 increased from baseline to week 12 by a least-squares (LS) mean of 160 ml (95% CI, 126 to 195) with dupilumab and 77 ml (95% CI, 42 to 112) with placebo (LS mean difference, 83 ml; 95% CI, 42 to 125; P<0.001), a difference that was sustained through week 52. At week 52, the SGRQ score had improved by an LS mean of -9.7 (95% CI, -11.3 to -8.1) with dupilumab and -6.4 (95% CI, -8.0 to -4.8) with placebo (LS mean difference, -3.4; 95% CI, -5.5 to -1.3; P = 0.002). The E-RS-COPD score at week 52 had improved by an LS mean of -2.7 (95% CI, -3.2 to -2.2) with dupilumab and -1.6 (95% CI, -2.1 to -1.1) with placebo (LS mean difference, -1.1; 95% CI, -1.8 to -0.4; P = 0.001). The numbers of patients with adverse events that led to discontinuation of dupilumab or placebo, serious adverse events, and adverse events that led to death were balanced in the two groups
- Conclusions: Among patients with COPD who had type 2 inflammation as indicated by elevated blood eosinophil counts, those who received dupilumab had fewer exacerbations, better lung function and quality of life, and less severe respiratory symptoms than those who received placebo
- Dupilumab for COPD with Blood Eosinophil Evidence of Type 2 Inflammation. N Engl J Med. 2024 May 20. doi: 10.1056/NEJMoa2401304 [MEDLINE]
- Background: Dupilumab, a fully human monoclonal antibody that blocks the shared receptor component for interleukin-4 and interleukin-13, key and central drivers of type 2 inflammation, has shown efficacy and safety in a phase 3 trial involving patients with chronic obstructive pulmonary disease (COPD) and type 2 inflammation and an elevated risk of exacerbation. Whether the findings would be confirmed in a second phase 3 trial was unclear
- Methods: In a phase 3, double-blind, randomized trial, we assigned patients with COPD who had a blood eosinophil count of 300 cells per microliter or higher to receive subcutaneous dupilumab (300 mg) or placebo every 2 weeks. The primary end point was the annualized rate of moderate or severe exacerbations. Key secondary end points, analyzed in a hierarchical manner to adjust for multiplicity, included the changes from baseline in the prebronchodilator forced expiratory volume in 1 second (FEV1) at weeks 12 and 52 and in the St. George’s Respiratory Questionnaire (SGRQ; scores range from 0 to 100, with lower scores indicating better quality of life) total score at week 52
- Results: A total of 935 patients underwent randomization: 470 were assigned to the dupilumab group and 465 to the placebo group. As prespecified, the primary analysis was performed after a positive interim analysis and included all available data for the 935 participants, 721 of whom were included in the analysis at week 52. The annualized rate of moderate or severe exacerbations was 0.86 (95% confidence interval [CI], 0.70 to 1.06) with dupilumab and 1.30 (95% CI, 1.05 to 1.60) with placebo; the rate ratio as compared with placebo was 0.66 (95% CI, 0.54 to 0.82; P<0.001). The prebronchiodilator FEV1 increased from baseline to week 12 with dupilumab (least-squares mean change, 139 ml [95% CI, 105 to 173]) as compared with placebo (least-squares mean change, 57 ml [95% CI, 23 to 91]), with a significant least-squares mean difference at week 12 of 82 ml (P<0.001) and at week 52 of 62 ml (P = 0.02). No significant between-group difference was observed in the change in SGRQ scores from baseline to 52 weeks. The incidence of adverse events was similar in the two groups and consistent with the established profile of dupilumab
- Conclusions: In patients with COPD and type 2 inflammation as indicated by elevated blood eosinophil counts, dupilumab was associated with fewer exacerbations and better lung function than placebo.
Theophylline (see Theophylline)
Pharmacology
- xxxx
Clinical Efficacy
- Benefit of Theophylline in COPD is Unclear (Chest, 2001) [MEDLINE]
Recommendation (American College of Chest Physicians/Canadian Thoracic Society Guidelines on the Prevention of COPD Exacerbations, 2015) (Chest, 2015) [MEDLINE]
- In Stable COPD, Oral Slow-Release Theophylline BID is Recommended to Decrease the COPD Exacerbation Rate (Grade 2B Recommendation)
Roflumilast (Daliresp, Daxas) (see Roflumilast)
- Contraindications
- Concomitant Theophylline Use (see Theophylline)
- Depression (see Depression): roflumilast should be used with caution in the setting of depression
- Pharmacology: phosphodiesterase type 4 inhibitor (PDE4 Inhibitor) (see Phosphodiesterase Type 4 Inhibitors)
- Anti-Inflammatory Effect
- Administration: 500 ug PO qday
- Adverse Effects
- Anorexia (see Anorexia)
- Back Pain (see Back Pain)
- Diarrhea (see Diarrhea)
- Insomnia (see Insomnia)
- Nausea (see Nausea and Vomiting)
- Weight Loss (see Weight Loss)
- Clinical Efficacy
- Cardiovascular Safety of Roflumilast in COPD (Chest, 2013) [MEDLINE]
- Roflumilast Decreased the Rates of Non-Fatal Acute MI and Non-Fatal Stroke
- REACT Trial in Patients with Severe COPD and Chronic Bronchitis Who are at Risk of Frequent and Severe Exacerbations Despite Inhaled Corticosteroids/LABA/Tiotropium (Lancet, 2015) [MEDLINE]
- Roflumilast Decreased Exacerbations and Hospital Admissions
- Cardiovascular Safety of Roflumilast in COPD (Chest, 2013) [MEDLINE]
- Recommendation (American College of Chest Physicians/Canadian Thoracic Society Guidelines on the Prevention of COPD Exacerbations, 2015) (Chest, 2015) [MEDLINE]
- In Moderate-Severe COPD with Chronic Bronchitis and a History of ≥1 COPD Exacerbation in the Prior Year, Roflumilast is Recommended to Decrease the COPD Exacerbation Rate (Grade 2A Recommendation)
Mepolizumab (Nucala) (see Mepolizumab)
Clinical Efficacy
- METREX and METREO Trials of Mepolizumab in Eosinophilic COPD (NEJM, 2017) [MEDLINE]
- Mepolizumab at a dose of 100 mg was associated with a lower annual rate of moderate or severe exacerbations than placebo among patients with COPD and an eosinophilic phenotype
- This finding suggests that eosinophilic airway inflammation contributes to COPD exacerbations
- MATINEE Trial of Mepolizumab in Chronic Obstructive Pulmonary Disease
- GSK announces positive results from phase III trial of Nucala (mepolizumab) in COPD. News release. GSK. September 6, 2024 [LINK]
Opioids (see Opioids)
Indications
- Refractory Dyspnea (see Dyspnea)
Clinical Efficacy
- Trial of Opiates in Advanced COPD (CMAJ Open, 2013) [MEDLINE]
- Opioids Were a Helpful and Acceptable Intervention for Improving Dyspnea and Health-Related Quality of Life in Advanced COPD
- Adverse Effects were Minimal
Recommendations (Canadian Thoracic Society Guidelines for the Management of Dyspnea in COPD) (Can Respir J, 2011)* [MEDLINE]
- Oral (But Not Nebulized) Opiates are Recommended for the Treatment of refractory Dyspnea in Advanced COPD (Grade 2C Recommendation)
Metoprolol (see Metoprolol)
Clinical Efficacy
- BLOCK-COPD Trial of Metoprolol for the Prevention of Acute Exacerbations of Chronic Obstructive Pulmonary Disease (COPD) (NEJM, 2019) [MEDLINE]: n = 532
- Methods
- Mean (± SD) Age of the Patients was 65.0 ± 7.8 y/o
- Mean Forced Expiratory Volume in 1 Second (FEV1) was 41.1 ± 16.3% of the Predicted Value
- Trial was Stopped Early Because of Futility with Respect to the PrimaryEnd Point and Safety Concerns
- Results
- No Significant Between-Group Difference in the Median Time Until the First Exacerbation, Which was 202 Days in the Metoprolol Group and 222 Days in the Placebo Group (Hazard Ratio for Metoprolol vs Placebo 1.05; 95% CI: 0.84-1.32; P = 0.66)
- Metoprolol was Associated with an Increased Risk of Exacerbation Leading to Hospitalization (Hazard Ratio 1.91; 95% CI: 1.29-2.83)
- Frequency of Side Effects that were Possibly Related to Metoprolol was Similar in the Two Groups, as was the Overall Rate of Non-Respiratory Serious Adverse Events
- During the Treatment Period, There were 11 Deaths in the Metoprolol Group and 5 in the Placebo Group
- Conclusions
- Among patients with moderate or severe COPD who did not have an established indication for beta-blocker use, the time until the first COPD exacerbation was similar in the metoprolol group and the placebo group
- Hospitalization for exacerbation was more common among the patients treated with metoprolol
- Methods
Bronchoscopic Lung Volume Reduction (see xxxx)
Endobronchial Valve Therapy
- Product
- Zephyr Endobronchial Valve (Emphasys Medical/Pulmonyx)
- Technique
- Duckbill valve mechanism placed unilaterally via bronchoscopy
- Clinical Efficacy
- Modest Improvement in Symptoms/Lung Function After Zephyr Placement: best candidates are probably those with intact inter lobar fissures and no collateral ventilation
- Exclusion Criteria: FEV1 <20% pred, hypercapnia, pulmonary hypertension, DLCO <25% pred
- Endobronchial Valve (Zephyr) for Emphysema Palliation Trial (VENT) Trial (NEJM, 2010) [MEDLINE]: randomized multi-center trial of endobronchial valves in heterogeneous emphysema (n = 321)
- At 90 Days
- Increased Rate of COPD Exacerbation (Requiring Hospitalization) and Hemoptysis
- At 6 mo
- Modest Improvement in FEV1 and 6MWT Distance, with a Non-Statistically-Significant Increase in Mortality (2.8% vs 0% in Control Group, p = 0.19)
- At 12 mo
- Valve Group Had 4.2% Pneumonia Rate in the Target Lobe, Bit No Change in Complication Rates or Mortality
- At 90 Days
- Retrospective Analysis from Multicenter Registry
- Dutch STELVIO Trial of Endobronchial Valves (from Pulmonyx) in Patients without Interlobar Collateral Ventilation (NEJM, 2015) [MEDLINE]
- At 6 mo, Endobronchial Valves Improved PFT’s and 6MWT Distance in Patients Without Interlobar Collateral Ventilation (As Assessed by Complete Fissure on HRCT)
- Adverse Event Rate was Higher (with 1 Death in the Valve Group)
- Modest Improvement in Symptoms/Lung Function After Zephyr Placement: best candidates are probably those with intact inter lobar fissures and no collateral ventilation
Intrabronchial Valve Therapy
- Product: Spiration implantable Intrabronchial Valve (Spiration)
- Technique: umbrella-shaped nitinol frame with synthetic polymer cover placed through flexible bronchoscope
- Sustained clinical benefits of spiration valve system in severe emphysema patients: 24-month follow-up of EMPROVE. Ann Am Thorac Soc. 2023 Nov 10. doi: 10.1513/AnnalsATS.202306-520OC [MEDLINE]
- Rationale: Follow-up of emphysema patients treated with endobronchial valves is limited to 3-12 months after treatment in prior reports. To date, no comparative data exist between treatment and controls with a longer follow-up
- Objective: To assess the durability of the Spiration® Valve System (SVS) in patients with severe heterogeneous emphysema over a 24-month period
- Methods: EMPROVE, a multicenter, randomized controlled trial, presents a rigorous comparison between treatment and control groups for up to 24 months. Lung function, respiratory symptoms, and quality-of-life (QOL) measures were assessed
- Results: A significant improvement in forced expiratory volume in 1 second was maintained at 24 months in the SVS treatment vs. control group. Similarly, significant improvements were maintained in several QOL measures, including St. George’s Respiratory Questionnaire and the COPD Assessment Test. Patients in the SVS treatment group experienced significantly less dyspnea than those in the control group, as indicated by the modified Medical Research Council Dyspnea Scale score. Adverse events at 24 months did not significantly differ between the SVS treatment and control groups. Acute COPD exacerbation rates in the SVS treatment and control groups were 13.7% (14/102) and 15.6% (7/45), respectively. Pneumothorax rates in the SVS treatment and control groups were 1.0% (1/102) and 0.0% (0/45), respectively
- Conclusions: SVS treatment resulted in statistically significant and clinically meaningful durable improvements in lung function, respiratory symptoms, and QOL, as well as a statistically significant reduction in dyspnea, for at least 24 months, while maintaining an acceptable safety profile.
Nitinol Coil Therapy
- Technique spring-like device delivered via bronchoscope
- Clinical Efficacy
- French REVOLENS Trial of Nitinol Coils (JAMA, 2016) [MEDLINE]
- Approximately 66% of Trial Patients Had Homogenous Emphysema (Generally Considered to Be Not Amenable to Surgery or Endobronchial Valve Placement)
- Compared to Usual Care, Bronchoscopic Treatment with Nitinol Coils Resulted in Improved Exercise Capacity with High Short-Term Costs: incremental cost-effectiveness ratio was $782,598 per additional quality-adjusted life-year
- French REVOLENS Trial of Nitinol Coils (JAMA, 2016) [MEDLINE]
Biologic Lung Volume Reduction Therapy
- Technique: application of sealant/remodeling system to collapse areas of emphysematous lung
Thermal Airway Ablation Therapy
- Technique: steam vapor applied to segmental airways
Airway Bypass Procedure
- Technique: extra-anatomic bronchial fenestration is used to decompress areas of emphysema (stent placed through bronchial wall into an area with severe emphysema decompresses the emphysematous area)
Bronchoscopic Vapor Ablation Lung Volume Reduction
- Technique
- Bronchoscopic application of thermal vapor ablation
- Clinical Efficacy
- STEP-UP Trial of Bronchoscopic Vapor Ablation Lung Volume Reduction in Emphysema (Lancet Respir Med, 2016) [MEDLINE]
- Vapor Ablation of More Diseased Lung Segments (with Preservation of Less Diseased Segments) Improved Lung Function (FEV1) and Quality of Life at 6 mo
- Acceptable Safety Profile: most common serious adverse event was COPD exacerbation, one death was reported in treatment group
- STEP-UP Trial of Bronchoscopic Vapor Ablation Lung Volume Reduction in Emphysema (Lancet Respir Med, 2016) [MEDLINE]
Lung Volume Reduction
Indications
- Maximal Exercise Tolerance <50W
Technique
- Lung Volume Reduction Surgery (LVRS)
- Operative Mortality: <10% (in experienced centers)
- Bronchoscopic Lung Volume Reduction
- XXXX
Current Criteria for Ideal Candidates for LVRS
- General Comments
- 50% of these candidates demonstrate significant improvement in lung function post-operatively
- Predominantly Upper Lobe Disease
- Absence of Significant Pulmonary Hypertension
- Absence of Hypercapnia
- FEV1 >20% Pred with DLCO >20% Pred
Clinical Efficacy
- Improved Mortality
- Improved Symptoms
- Improved Lung Function/Functional Status
- Improved BMI (Am J Respir Crit Care Med, 2012) [MEDLINE]: especially in COPD patients who had prior low BMI
- The Improvement in BMI is Associated with Improvements in Lung Function, Exercise Capacity, Respiratory Muscle Strength, and Ventilatory Efficiency
- Study of Bronchoscopic Lung Volume Reduction with Coils/Endobronchial Valves (Respir Med, 2022) [MEDLINE]: n = 1,471
- A total of 531 patients (35%) died during follow-up and the median survival time of the total population was 2694 days (95% confidence interval(CI) 2462-2926) which is approximately 7.4 years
- The median survival time of patients who were treated with BLVR was significantly longer compared to patients who were not treated with bronchoscopic lung volume reduction (3133 days versus 2503 days, p < 0.001), and bronchoscopic lung volume reduction was found to be an independent predictor of survival when adjusting for other survival-influencing factors such as age, gender or severity of disease
- Bronchoscopically reducing lung volume in patients with severe hyperinflation may lead to a survival benefit for a population with a severely reduced life expectancy
- Sustained clinical benefits of Spiration Valve System in patients with severe emphysema: 24-Month follow-up of EMPROVE. Ann Am Thorac Soc. February 2024;21(2):251-260. doi:10.1513/AnnalsATS.202306-520OC [MEDLINE]
- Rationale: Follow-up of patients with emphysema treated with endobronchial valves is limited to 3-12 months after treatment in prior reports. To date, no comparative data exist between treatment and control subjects with a longer follow-up. Objectives: To assess the durability of the Spiration Valve System (SVS) in patients with severe heterogeneous emphysema over a 24-month period
- Methods: EMPROVE, a multicenter randomized controlled trial, presents a rigorous comparison between treatment and control groups for up to 24 months. Lung function, respiratory symptoms, and quality-of-life (QOL) measures were assessed
- Results: A significant improvement in forced expiratory volume in 1 second was maintained at 24 months in the SVS treatment group versus the control group. Similarly, significant improvements were maintained in several QOL measures, including the St. George’s Respiratory Questionnaire and the COPD Assessment Test. Patients in the SVS treatment group experienced significantly less dyspnea than those in the control group, as indicated by the modified Medical Research Council dyspnea scale score. Adverse events at 24 months did not significantly differ between the SVS treatment and control groups. Acute chronic obstructive pulmonary disease exacerbation rates in the SVS treatment and control groups were 13.7% (14 of 102) and 15.6% (7 of 45), respectively. Pneumothorax rates in the SVS treatment and control groups were 1.0% (1 of 102) and 0.0% (0 of 45), respectively
- Conclusions: SVS treatment resulted in statistically significant and clinically meaningful durable improvements in lung function, respiratory symptoms, and QOL, as well as a statistically significant reduction in dyspnea, for at least 24 months while maintaining an acceptable safety profile.
Bullectomy
Criteria for Ideal Candidates for Bullectomy
- Bulla is >1/3 of Hemithorax
- The rare bulla which communicates with airways (as determined by disparity between body box and helium dilution TLC) is more likely to lead to improvement with bullectomy (as it contributes more to dead space ventilation, worsening dyspnea)
- Absence of Generalized Emphysematous Changes in Remaining Lung: the best test for this is a inspiratory/expiratory chest CT scan
- Presence of generalized emphysema predicts poor results
- FEV1 Around 50% Predicted: most benefit in this group
- Those with higher FEV1 generally have few symptoms (with little room for improvement)
- Those with lower FEV1 generally have generalized emphysema
Postoperative Outcome from Bullectomy
- Prediction of Post-Op Outcomes: bronchography, exercise testing, and quantitative V/Q scanning do not predict post-operative outcome from bullectomy
- Post-Op Outcomes
- Most patients without severe emphysema show improved FEV1 and FVC post-operatively
- DLCO is improved, if the compressed lung is better perfused
- Post-op ventilatory capacity and oxygen consumption (at anaerobic threshold and maximal exercise) are both improved
Noninvasive Positive Pressure Ventilation (NIPPV)
- German/Austrian Randomized Trial of NIPPV in Stable Hypercapnic COPD (2014) [MEDLINE]
- Study: randomized German/Austrian trial of NIPPV (n = 195 from 36 centers)
- NIPPV was used for at least 6 hrs per day (preferably during sleep)
- NIPPV was provided using ventilator set to pressure support mode with a backup rate, or alternately assisted ventilation (if high backup rates were not tolerated)
- NIPPV was targted to decrease pCO2 by at least 20%
- Main Findings: addition of long-term NIPPV to standard COPD treatment improved survival of patients with hypercapnic, stable COPD when the NIPPV is targeted to significantly reduce hypercapnia (1-year mortality was 12% in the NIPPV group vs 33% in the control group; hazard ratio 0.24 (95% CI 0.11-0.49; p=0.0004)
- Study: randomized German/Austrian trial of NIPPV (n = 195 from 36 centers)
- Systematic Review and Meta-Analysis of Home Noninvasive Positive Pressure Ventilation in Patients with Hypercapnic Chronic Obstructive Pulmonary Disease (JAMA, 2020) [MEDLINE]: 21 RCT’s and 12 observational studies (n = 51, 085
- Study Population
- Mean Age was 65.7 +/- 2.1 y/o (SD)
- Sex: 43% female were included
- 434 Deaths
- 27 Patients Who Underwent Intubation
- BPAP compared with no device was significantly associated with lower risk of mortality (22.31% vs 28.57%; risk difference [RD], -5.53% [95% CI, -10.29% to -0.76%]; odds ratio [OR], 0.66 [95% CI, 0.51-0.87]; P = .003; 13 studies; 1423 patients; strength of evidence [SOE], moderate), fewer patients with all-cause hospital admissions (39.74% vs 75.00%; RD, -35.26% [95% CI, -49.39% to -21.12%]; OR, 0.22 [95% CI, 0.11-0.43]; P < .001; 1 study; 166 patients; SOE, low), and lower need for intubation (5.34% vs 14.71%; RD, -8.02% [95% CI, -14.77% to -1.28%]; OR, 0.34 [95% CI, 0.14-0.83]; P = .02; 3 studies; 267 patients; SOE, moderate)
- Study Population
CONCLUSIONS AND RELEVANCE:
- In this meta-analysis of patients with COPD and hypercapnia, home BPAP, compared with no device, was associated with lower risk of mortality, all-cause hospital admission, and intubation, but no significant difference in quality of life
- Noninvasive HMV, compared with no device, was significantly associated with lower risk of hospital admission, but there was no significant difference in mortality risk
- However, the evidence was low to moderate in quality, the evidence on quality of life was insufficient, and the analyses for some outcomes were based on small numbers of studies.
- Macrea M, Oczkowski S, Rochwerg B, et al. Long-term noninvasive ventilation in chronic stable hypercapnic chronic obstructive pulmonary disease. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;202(4):e74-e87. doi:10.1164/rccm.202006-2382ST [MEDLINE]
- McCormick JL, Clark TA, Shea CM, et al. Exploring the patient experience with noninvasive ventilation: a human-centered design analysis to inform planning for better tolerance. Chronic Obstr Pulm Dis. 2022;9(1):80-94. doi: 10.15326/jcopdf.2021.0274 [MEDLINE]
- Yu DSF, Li PWC, Lau JCC, et al. Health communication and adherence to noninvasive ventilation in chronic hypercapnic respiratory failure: a randomized clinical trial. JAMA Netw Open. 2024;7(12):e2451614. doi:10.1001/jamanetworkopen.2024.51614 [MEDLINE]
- Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5(2):173-178. PMID: 18250209; PMCID: PMC2645251 [MEDLINE]
- doi: 10.1513/pats.200708-119MGSweetman A, Lack L, Catcheside PG, et al. Cognitive and behavioral therapy for insomnia increases the use of continuous positive airway pressure therapy in obstructive sleep apnea participants with comorbid insomnia: a randomized clinical trial. Sleep. 2019;42(12):zsz178. PMID: 31403168. doi: 10.1093/sleep/zsz178 [MEDLINE]
- Genta PR, Kaminska M, Edwards BA, et al. The importance of mask selection on continuous positive airway pressure outcomes for obstructive sleep apnea. An official American Thoracic Society workshop report. Ann Am Thorac Soc. 2020;17(10):1177-1185. doi:10.1513/AnnalsATS.202007-864ST [MEDLINE]
- Kaminska M, Adam V, Orr JE. Home noninvasive ventilation in COPD. Chest. 2024;165(6):1372-1379. doi:10.1016/j.chest.2024.01.030 [MEDLINE]
Nutritional Management
- Despite prevalence of cachexia in COPD, there is no evidence that enhanced nutrition improves body wieght, lung function, exercise capacity, or survival
Management of Dyspnea (in Advanced COPD) (see Dyspnea)
- Recommendations (Canadian Thoracic Society Guidelines for the Management of Dyspnea in COPD) (Can Respir J, 2011) [MEDLINE]
- Anxiolytics/Antidepressants are Not Routinely Recommended for the Management of Dyspnea in Advanced COPD (Grade 2B Recommendation)
- Oral (But Not Nebulized) Opiates are Recommended for the Treatment of refractory Dyspnea in Advanced COPD (Grade 2C Recommendation)
- Neuromuscular Electrical Muscle Stimulation (NMES) and Chest Wall Vibration are Recommended to Decrease Dyspnea in Advanced COPD
- Walking Aids are Recommended for Appropriate Patients to Decrease Dyspnea in Advanced COPD (Grade 2B Recommendation)
- Pursed-Lip Breathing is Recommended to Decrease Dyspnea in Advanced COPD (Grade 2B Recommendation)
- Insufficient Evidence to Recommend the Routine Use of Acupuncture, Acupressure, Distractive Auditory Stimuli (Music), Relaxation, Handheld Fans, Counseling and Support Programs, or Psychotherapy to Decrease Dyspnea in Advanced COPD
- Continuous Oxygen Therapy for Hypoxemic Patients Decreases the Mortality Rate and May Decrease Dyspnea in Advanced COPD (Grade 2B Recommendation) (see Oxygen)
- No Evidence to Support the Routine Use of Supplemental Oxygen to Decrease Dyspnea in Normoxemic Patients with Advanced COPD
- There is Little Benefit from Supplemental Oxygen on Quality of Life in Patients with Advanced COPD
Treatment of Concomitant Metabolic Alkalosis (see Metabolic Alkalosis)
Clinical Efficacy
- Trial of Acetazolamide to Treat Metabolic Alkalosis in the Setting of Chronic Obstructive Pulmonary Disease (COPD) or Obesity Hypoventilation Syndrome (OHS) (Respir Care, 2010) [MEDLINE]
- Acetazolamide Decreased Serum Bicarbonate and Increased Carbon Dioxide Responsiveness
- Systematic Review of Acetazolamide in the Treatment of Metabolic Alkalosis Complicating Chronic Hypercapnic Respiratory Failure in Chronic Obstructive Pulmonary Disease (COPD) or Obesity Hypoventilation Syndrome (Thorax, 2023) [MEDLINE]: n = 504 (4 studies) (99% of patients in study had chronic obstructive pulmonary disease; 50% of trials recruited patients requiring mechanical ventilation)
- Rationale
- Metabolic Alkalosis May Lead to Respiratory Inhibition and Increase the Need for Ventilatory Support (or Prolong Ventilator Weaning) for Patients with Chronic Respiratory Disease
- Acetazolamide Can Decrease Alkalemia and May Decrease Respiratory Depression
- Primary Outcome
- Mortality Rate
- Risk of Bias: low-some risk
- There was No Statistically Significant Difference with Acetazolamide with Respect to Mortality Rate (Relative Risk 0.98; 95% CI: 0.28 to 3.46); p = 0.95; 490 Subjects; Three Studies; GRADE: Low Certainty) or Duration of Ventilatory Support (Mean Difference -0.8 days; 95% CI -7.2 to 5.6; p = 0.36; 427 Subjects; Two Studies; GRADE: Low Certainty)
- Clinically Significant Benefits or Harms are Unable to Be Excluded, and Larger Trials are Required
- Rationale
Management of Pulmonary Hypertension (see Pulmonary Hypertension)
- Calcium Channel Blockers (see Calcium Channel Blockers): Nifedipine decreases exercise PA pressure and CO (effects last up to 9 weeks but symptoms are unchanged)
- Verapamil is not an effective pulmonary vasodilator in COPD
- Prazosin (see Prazosin): decreases PA pressure and increases CO in COPD (effects last for 8 weeks but cause a decrease in pO2 and worsened dyspnea)
Treatment of Polycythemia (see Polycythemia)
- If patient is polycythemic, treatment decreases PA pressure, decreases PVR, and increases RV-EF
Scuba Diving with Chronic Obstructive Pulmonary Disease
- Contraindicated: due to risk of pneumothorax
End of Life Care
- xxx
- Differences in Health Care and Palliative Care Use at the End of Life: A Comparison Study Among Lung Cancer, COPD, and Idiopathic Pulmonary Fibrosis. Chest. 2024 Dec;166(6):1487-1496. doi: 10.1016/j.chest.2024.08.018 [MEDLINE]
- Background: Patients with lung cancer, idiopathic pulmonary fibrosis (IPF), and COPD have high symptom burden, poor quality of life, and high health care use at the end of life. Although proactive integration of palliative care in lung cancer can improve outcomes, it is unclear whether similar practices have been adopted in COPD and IPF care
- Research question: Do patients with COPD and IPF have different patterns of health care and palliative care use at the end of life compared with patients with lung cancer?
- Study design and methods: We retrospectively identified deceased patients with lung cancer, COPD, or IPF with ≥ 1 outpatient visit at the University of California, San Francisco, in the last 6 months of life. We compared outpatient palliative care and opioid prescriptions, inpatient palliative care, hospitalizations, intensive care use, and in-hospital death in the last 6 months of life between each group. We used multivariable logistic regression to calculate adjusted ORs (aORs) of each outcome, with lung cancer as the reference group
- Results: Among 1,819 patients, patients with COPD and IPF were more likely to be male and older at the time of death compared with patients with lung cancer. Compared with patients with lung cancer, patients with COPD and IPF showed a lower adjusted odds (P < .001) of receiving outpatient palliative care (COPD: aOR, 0.26 [95% CI, 0.19-0.36]; IPF: aOR, 0.48 [95% CI, 0.32-0.70]), outpatient opioid prescription (COPD: aOR, 0.50 [95% CI, 0.40-0.63]; IPF: aOR, 0.40 [95% CI, 0.29-0.54]), and a higher odds of end-of-life ICU use (COPD: aOR, 2.88 [95% CI, 2.11-3.93]; IPF: aOR, 4.15 [95% CI, 2.66-6.49]). Patients with IPF showed higher odds of receiving inpatient palliative care (aOR: 2.02 [95% CI, 1.30-3.13]; P = .002) Interpretation: This study showed that patients with COPD and IPF are less likely to receive outpatient palliative care and opioid prescriptions and are more likely to use end-of-life intensive care than patients with lung cancer. Further research should explore health system barriers contributing to differences in care patterns to optimize quality of life and to align with patient goals of care.
