Clinical Manifestations

General Comments
Clinical Indicators Which May Signal the Presence of Chronic Obstructive Pulmonary Disease (COPD) (GOLD; Global Strategy for Diagnosis, Management, and Prevention of COPD, 2016) [LINK]
- Dyspnea (see Dyspnea)
- Progressive
- Characteristically Worse with Exercise
- Persistent
- Chronic Cough (see Cough)
- May Be Intermittent
- May Be Dry
- Chronic Sputum Production
- Any Pattern
- History of Exposures
- Tobacco Smoke (see Tobacco)
- Smoke from Home Cooking and Heating Fuels
- Occupational Dusts and Exposures
- Family History of COPD
- Patient Burden and Insights in COPD: A Survey Analysis. Chronic Obstr Pulm Dis. 2025 Jul 30;12(4):317-327. doi: 10.15326/jcopdf.2025.0616 [MEDLINE]
- Background: Chronic obstructive pulmonary disease (COPD) affects millions of people and is associated with significant morbidity and mortality. Patients experience a high symptom burden with impacts on quality of life, which have not been well quantified
- Methods: Phreesia’s PatientInsightsquantitative survey was offered in January 2025 to patients with COPD during their check-in process for health care provider (HCP) visits. The survey comprised 28 questions. Survey question categories included COPD symptom experience and impact, and the treatment journey of patients with COPD. The survey also sought to identify potential communication gaps between patients and HCPs that might hinder effective COPD management
- Results: Of 1615 patients surveyed, most (59%) were female, and the majority identified as White (82%). A total of 39% of patients had experienced COPD for over 7 years at the time the survey was conducted, and 25% reported experiencing symptoms all 30 days in a typical month. A large proportion (64%) said that COPD had a moderate-to-great impact on their daily lives. Only 45% of patients had detailed discussions about their COPD with their HCPs. Among patients who had not tried/were currently not on any maintenance medications (n=339), the leading reasons included that their COPD was not severe enough, and that their HCP had not recommended it. Among patients who had tried maintenance medications, the majority (77%) indicated that they would be willing to try another therapy
- Conclusions: Improvements in patient-HCP communication are needed to achieve more effective, timely COPD management.
Cardiovascular Manifestations
Cardiac Arrhythmias
- Epidemiology
- COPD is Associated with Increased Cardiovascular Morbidity and Mortality
- Physiology
- May Be Related to Hypoxemia (Especially During REM Sleep) or to Other Factors
- Clinical
- Atrial Fibrillation (AF) (see Atrial Fibrillation)
- Multifocal Atrial Tachycardia (MAT) (see Multifocal Atrial Tachycardia)
- Ventricular Tachycardia (VT) (see Ventricular Tachycardia)
- Clinical Data
- Even After Adjusting for Age/Gender/Tobacco Use/Obesity/Hypertension/CAD/Heart Failure/Diabetes Mellitus/Anemia/Cancer/CKD/Rate or Rhythm Control Medications, COPD was a Significant Risk Factor for Atrial Fibrillation/Flutter, and Non-Sustained/Sustained Ventricular Tachycardia (Am J Cardiol, 2014) [MEDLINE]
Increased Risk of Acute Myocardial Infarction (see Coronary Artery Disease)
- Epidemiology
- COPD is Associated with an Increased Risk for Cardiovascular Disease, Stroke, and Diabetes Mellitus (Thorax, 2010) [MEDLINE]
- After Adjusting for Sex/Smoking Status/Age, the Greatest Risk for Arteriovascular Events was Observed in the Youngest Age Group
- Hazard Ratio for Acute MI was 10.34 (95% CI 3.28 to 32.60; p<0.001) in the Youngest Age Group, as Compared to the Oldest Age Group
- COPD is Associated with an Increased Risk for Cardiovascular Disease, Stroke, and Diabetes Mellitus (Thorax, 2010) [MEDLINE]
- Sex-specific and age-specific incidence of ischaemic heart disease, atrial fibrillation and heart failure in community patients with chronic obstructive pulmonary disease. BMJ Open Respir Res. 2022 Dec;9(1):e001307. doi: 10.1136/bmjresp-2022-001307 [MEDLINE]
- Objective: To estimate the incidence of ischaemic heart disease, atrial fibrillation and heart failure in community patients with or without chronic obstructive pulmonary disease (COPD)
- Methods: For this population-based study, we used primary care data of the Julius General Practitioners’ Network. Eligible participants were aged 40-80 years old and contributed data between January 2014 and February 2019. Participants were divided into groups according to COPD status and were followed up for new ischaemic heart disease, atrial fibrillation and/or heart failure. Age-specific and sex-specific incidence and incidence rate ratios were calculated for patients with and without COPD
- Results: Mean follow-up was 3.9 years, 6223 patients were included in the COPD group, and 137 028 individuals in the background group without COPD. Incidence rates of all three heart diseases increased with age and were higher in males, independent of presence of COPD. Incidence rate ratios for patients with COPD, adjusted for age and sex, were 1.69 (95% CI 1.49 to 1.92) for ischaemic heart disease, 1.56 (95% CI 1.38 to 1.77) for atrial fibrillation and 2.96 (95% CI 2.58 to 3.40) for heart failure
- Conclusion: The incidence of all major cardiovascular diseases is higher in patients with COPD, with the highest incidence rate ratio observed for heart failure.
- Impaired Spirometry and COPD Increase the Risk of Cardiovascular Disease: A Canadian Cohort Study. Chest. 2023 Sep;164(3):637-649. doi: 10.1016/j.chest.2023.02.045 [MEDLINE]
- Background: Individuals with COPD and preserved ratio impaired spirometry (PRISm) findings in clinical settings have an increased risk of cardiovascular disease (CVD)
- Research question: Do individuals with mild to moderate or worse COPD and PRISm findings in community settings have a higher prevalence and incidence of CVD compared with individuals with normal spirometry findings? Can CVD risk scores be improved when impaired spirometry is added?
- Study design and methods: The analysis was embedded in the Canadian Cohort Obstructive Lung Disease (CanCOLD). Prevalence of CVD (ischemic heart disease [IHD] and heart failure [HF]) and their incidence over 6.3 years were compared between groups with impaired and normal spirometry findings using logistic regression and Cox models, respectively, adjusting for covariables. Discrimination of the pooled cohort equations (PCE) and Framingham risk score (FRS) in predicting CVD were assessed with and without impaired spirometry
- Results: Participants (n = 1,561) included 726 people with normal spirometry findings and 835 people with impaired spirometry findings (COPD Global Initiative for Chronic Obstructive Lung Disease [GOLD] stage 1 disease, n = 408; GOLD stage ≥ 2, n = 331; PRISm findings, n = 96). Rates of undiagnosed COPD were 84% in GOLD stage 1 and 58% in GOLD stage ≥ 2 groups. Prevalence of CVD (IHD or HF) was significantly higher among individuals with impaired spirometry findings and COPD compared with those with normal spirometry findings, with ORs of 1.66 (95% CI, 1.13-2.43; P = .01∗) (∗ indicates statistical significane with P < .05) and 1.55 (95% CI, 1.04-2.31; P = .033∗), respectively. Prevalence of CVD was significantly higher in participants having PRISm findings and COPD GOLD stage ≥ 2, but not GOLD stage 1. CVD incidence was significantly higher, with hazard ratios of 2.07 (95% CI, 1.10-3.91; P = .024∗) for the impaired spirometry group and 2.09 (95% CI, 1.10-3.98; P = .024∗) for the COPD group compared to individuals with normal spirometry findings. The difference was significantly higher among individuals with COPD GOLD stage ≥ 2, but not GOLD stage 1. The discrimination for predicting CVD was low and limited when impaired spirometry findings were added to either risk score
- Interpretation: Individuals with impaired spirometry findings, especially those with moderate or worse COPD and PRISm findings, have increased comorbid CVD compared with their peers with normal spirometry findings, and having COPD increases the risk of CVD developing
- Differential Association of COPD Subtypes With Cardiovascular Events and COPD Exacerbations. Chest. 2024 Dec;166(6):1360-1370. doi: 10.1016/j.chest.2024.07.148 [MEDLINE]
- Background: The coronary artery calcium score (CACS) and ratio of the pulmonary artery to aorta diameters (PA:A ratio) measured from chest CT scans have been established as predictors of cardiovascular events and COPD exacerbations, respectively. However, little is known about the reciprocal relationship between these predictors and outcomes. Furthermore, the prognostic implications of COPD subtypes on clinical outcomes remain insufficiently characterized
- Research question: How can these two chest CT scan-derived parameters predict subsequent cardiovascular events and COPD exacerbations in different COPD subtypes?
- Study design and methods: Using COPDGene study data, we assessed prospective cardiovascular disease (CVD) and COPD exacerbation risk in participants with COPD (Global Initiative for Chronic Obstructive Lung Disease spirometric grades 2-4), focusing on CACS and PA:A ratio at study enrollment, with logistic regression models. These outcomes were analyzed in three COPD subtypes: 1,042 participants with non-emphysema-predominant COPD (NEPD; low attenuation area at -950 Hounsfield units [LAA-950] < 5%), 1,324 participants with emphysema-predominant COPD (EPD; LAA-950 ≥ 10%), and 465 participants with intermediate emphysema COPD (IE; 5% ≤ LAA-950 < 10%)
- Results: Our study indicated significantly higher overall risk for cardiovascular events in participants with higher CACS (≥ median; OR, 1.61; 95% CI, 1.30-2.00) and increased COPD exacerbations in those with higher PA:A ratios (≥ 1; OR, 1.80; 95% CI, 1.46-2.23). Notably, participants with NEPD showed a stronger association between these indicators and clinical events compared to EPD (with CACS/CVD, NEPD vs EPD: OR, 2.02 vs 1.41; with PA:A ratio/COPD exacerbation, NEPD vs EPD: OR, 2.50 vs 1.65); the difference in ORs between COPD subtypes was statistically significant for CACS/CVD
- Interpretation: Two chest CT scan parameters, CACS and PA:A ratio, hold distinct predictive values for cardiovascular events and COPD exacerbations that are influenced by specific COPD subtypes.
Neurologic Manifestations
Anxiety/Depression (see Anxiety, [[Anxiety]] and Depression)
- Epidemiology
- Anxiety/Depression Occur with Higher Prevalence in COPD than in Other Chronic Diseases (Chest, 2008) [MEDLINE]
- Prevalence of Anxiety in COPD: 10-33%
- Prevalence of Depression in COPD: 10-40%
- Untreated/Undetected Anxiety and Depression May Increase Physical Disability, Morbidity, and Health Care Utilization (Chest, 2008) [MEDLINE]
- Anxiety/Depression Occur with Higher Prevalence in COPD than in Other Chronic Diseases (Chest, 2008) [MEDLINE]
Circadian Rhythm Disruption
- Epidemiology
- XXX
- Association of rest-activity circadian rhythm with chronic respiratory diseases, a cross-section survey from NHANES 2011-2014. Respir Med. 2023 Feb 6;209:107147. doi: 10.1016/j.rmed.2023.107147 [MEDLINE]
- A total of 7412 participants from the National Health and Nutrition Examination Survey (NHANES) 2011-2014 were included in this study. The rest-activity circadian rhythm indices were calculated using accelerometer data and were divided into quartiles to perform logistic regression.
- Results: Participants in the highest quartile of Relative amplitude (RA) had a lower prevalence of emphysema, chronic bronchitis and asthma, compared to those in the lowest quartile. Participants in the highest quartile of Intradaily variability (IV) was associated with a higher prevalence of emphysema relative to those in the lowest quartile. Compared to those in the lowest quartile, participants in the highest quartile of the average activity of the most active continuous 10-h period (M10) had a lower prevalence of emphysema. Additionally, compared to those in the lowest quartile of the average activity of the least active continuous 5-h period (L5) and L5 start time, participants in the highest quartile had a higher prevalence of asthma.
- Conclusions: This study demonstrated that in general US adult population, disrupted rest-activity circadian rhythm was associated with a higher prevalence of chronic respiratory diseases
Increased Risk of Ischemic Cerebrovascular Accident (CVA) (see Ischemic Cerebrovascular Accident)
- Epidemiology:
- COPD is Associated with an Increased Risk for Cardiovascular Disease, Stroke, and Diabetes Mellitus (Thorax, 2010) [MEDLINE]
- After Adjusting for Sex/Smoking Status/Age, the Greatest Risk for Arteriovascular Events was Observed in the Youngest Age Group
- Hazard Ratio for Stroke was 3.44 (95% CI 0.85 to 13.84; p<0.001) in the Youngest Age Group, as Compared to the Oldest Age Group
- COPD is Associated with an Increased Risk for Cardiovascular Disease, Stroke, and Diabetes Mellitus (Thorax, 2010) [MEDLINE]
Neuropsychiatric Consequences of Hypoxemia
- Epidemiology
- Frequency correlates with the degree of hypoxemia
Pulmonary Manifestations
General Clinical Features
- Accessory Muscle Use
- Chest Tightness
- Chronic Productive Cough (see Cough)
- Clinical Definition of Chronic Bronchitis: sputum production for ≥3 months in the last 2 consecutive years (in the absence of any other conditions that may explain it)
- Clinical
- Cough-Related Rib Fractures
- Post-Tussive Syncope (see Syncope)
- Clinical Data
- Study of Chronic Productive Cough in Smokers with Early COPD (COPD, 2014) [MEDLINE]: used Lung Health Study data
- Combination of Cough with Sputum was Associated with Increased Mortality Rate in Mild-Moderate COPD (After Adjustment for Age, Gender, Race, Smoking Status at Year 5, Pack-Years Smoked, Randomization Group, and Baseline FEV1)
- Study of Chronic Productive Cough in Smokers with Early COPD (COPD, 2014) [MEDLINE]: used Lung Health Study data
- Dyspnea (see Dyspnea)
- Epidemiology: common
- Hoover Sign (see Hoover Sign, [[Hoover Sign]]): paradoxic inward inspiratory movement of lower lateral rib cage
- Hypoxemia (see Hypoxemia)
- Cyanosis (see Cyanosis)
- Tachypnea (see Tachypnea)
- Wheezing (see Wheezing)
Mucous Plugging
- xx
- Airway Mucus Plugs on Chest Computed Tomography Are Associated with Exacerbations in COPD. Am J Respir Crit Care Med. 2024 Oct 29;211(5):814-822. doi: 10.1164/rccm.202403-0632OC [MEDLINE]
- Rationale/Objective: Acute exacerbations (AEs) of chronic obstructive pulmonary disease (COPD) are associated with significant morbidity and mortality. Whether mucus plugs are associated with prospective exacerbations has not been examined extensively
- Methods: Mucus plugs were visually-identified on baseline chest computed tomography (CT) scans from smokers with Global Initiative for Chronic Obstructive Lung Disease (GOLD) grades 2-4 COPD enrolled in two multicenter cohort studies: Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) and COPDGene. Associations between ordinal mucus plug score categories (0/1-2/≥3) and prospectively-ascertained AEs, defined as worsening respiratory symptoms requiring systemic steroids and/or antibiotics (moderate-to-severe) and/or ER/hospitalization (severe), were assessed using multivariable-adjusted zero-inflated Poisson regression; subjects were exacerbation-free at enrollment
- Results: Among 3,250 participants in COPDGene (mean±SD age 63.7±8.4 years, FEV1 50.6%±17.8% predicted, 45.1% female) and 1,716 participants in ECLIPSE (age 63.3±7.1 years, FEV1 48.3%±15.8% predicted, 36.2% female), 44.4% and 46.0% had mucus plugs, respectively. The incidence rates of AEs were 61.0 (COPDGene) and 125.7 (ECLIPSE) per 100 person-years. Relative to those without mucus plugs, the presence of 1-2 and ≥3 mucus plugs was associated with increased risk (adjusted rate ratio, aRR [95%CI]=1.07[1.05-1.09] and 1.15[1.1-1.2] in COPDGene; aRR=1.06[1.02-1.09] and 1.12[1.04-1.2] in ECLIPSE, respectively) for prospective moderate-to-severe AEs. The presence of 1-2 and ≥3 mucus plugs was also associated with increased risk for severe AEs during follow-up (aRR=1.05[1.01-1.08] and 1.09[1.02-1.18] in COPDGene; aRR=1.17[1.07-1.27] and 1.37[1.15-1.62] in ECLIPSE, respectively)
- Conclusion: CT-based mucus plugs are associated with an increased risk for future COPD AEs.
- Silent Airway Mucus Plugs in COPD and Clinical Implications. Chest. 2024 Nov;166(5):1010-1019. doi: 10.1016/j.chest.2023.11.033 [MEDLINE]
- Background: Airway mucus plugs are frequently identified on CT scans of patients with COPD with a smoking history without mucus-related symptoms (ie, cough, phlegm [silent mucus plugs])
- Research question: In patients with COPD, what are the risk and protective factors associated with silent airway mucus plugs? Are silent mucus plugs associated with functional, structural, and clinical measures of disease
- Study design and methods: We identified mucus plugs on chest CT scans of participants with COPD from the COPDGene study. The mucus plug score was defined as the number of pulmonary segments with mucus plugs, ranging from 0 to 18, and categorized into three groups (0, 1-2, and ≥ 3). We determined risk and protective factors for silent mucus plugs and the associations of silent mucus plugs with measures of disease severity using multivariable linear and logistic regression models
- Results: Of 4,363 participants with COPD, 1,739 had no cough or phlegm. Among the 1,739 participants, 627 (36%) had airway mucus plugs identified on CT scan. Risk factors of silent mucus plugs (compared with symptomatic mucus plugs) were older age (OR, 1.02), female sex (OR, 1.40), and Black race (OR, 1.93) (all P values < .01). Among those without cough or phlegm, silent mucus plugs (vs absence of mucus plugs) were associated with worse 6-min walk distance, worse resting arterial oxygen saturation, worse FEV1 % predicted, greater emphysema, thicker airway walls, and higher odds of severe exacerbation in the past year in adjusted models
- Interpretation: Mucus plugs are common in patients with COPD without mucus-related symptoms. Silent mucus plugs are associated with worse functional, structural, and clinical measures of disease. CT scan-identified mucus plugs can complement the evaluation of patients with COPD.
- Longitudinal changes in airway mucus plugs and FEV1 in COPD. N Engl J Med. 2025 May 15;392(19):1973-1975. doi:10.1056/NEJMc2502456 [MEDLINE]
- No abstract
Radiologic Emphysema
- xxx
- Longitudinal Tracking of Emphysema Holes at Noncontrast CT: Dynamic Patterns and Clinical Relationships. Radiology. 2025 Jul;316(1):e243239. doi: 10.1148/radiol.243239 [MEDLINE]
- Background
- Emphysema holes change longitudinally in various ways, but current CT measurements lack the ability to fully capture these changes beyond measuring the extent of emphysema
- Purpose To track emphysema holes longitudinally, group them according to their dynamics, and investigate their relationship with change in forced expiratory volume in 1 second (FEV1), disease progression, and mortality
- Materials and Methods
- In this secondary analysis, data from participants in the Korean Obstructive Lung Disease cohort study from June 2005 to October 2013 who completed baseline and 6-year follow-up CT with identical protocols were evaluated. Emphysema holes were identified and tracked using deep learning-based software and were grouped based on changes in diameter (in 2-mm increments) as increased in diameter (including both new and enlarged preexisting holes), stable, or decreased in diameter
- The percentage of hole volume in each group and its relationship with FEV1 decline were analyzed using multiple linear regression, and comparisons were made among the subsets of participants on the basis of emphysema progression or severity
- Overall survival according to the volume cutoff of the holes with increased diameter was compared using the log-rank test
- Results
- Among 108 participants (mean age, 63.4 years ± 6.7 [SD]; 104 male), 39 had emphysema progression (based on whether the change in low-attenuation area less than -950 HU [LAA-950] exceeded 3.7%). Enlarged preexisting holes were marginally associated with a greater decline in FEV1 (β = -.25, P = .049)
- Compared with those without emphysema progression, those with emphysema progression had a significantly greater percentage of hole volume and percentage of holes with increased diameter (7.7% vs 1.9% and 18.3% vs 6.2%, respectively; both P < .001), with most of the volume attributed to new holes
- Participants with severe disease or emphysema (FEV1 < 50% or LAA-950 ≥ 14%) had more holes with increased diameter (5.1% vs 2.4% [P = .02] and 6.7% vs 1.2% [P < .001], respectively) and new holes (3.8% vs 1.7% [P = .01] and 4.7% vs 1.1% [P < .001], respectively)
- Participants with 5% or greater volume of increased-diameter holes had worse overall survival (log-rank P < .001)
- Conclusion
- Emphysema hole-tracking results showed that a greater volume of holes that increased in diameter were related to change in FEV1, disease progression, and mortality.
- Background
- Emphysema at Baseline Low-Dose CT Lung Cancer Screening Predicts Death from Chronic Obstructive Pulmonary Disease and Cardiovascular Disease Up to 25 Years Later. Radiology. 2025 Sep;316(3):e250949. doi: 10.1148/radiol.250949 [MEDLINE]
- Background
- The prognostic value of baseline visual emphysema scoring at low-dose CT (LDCT) in lung cancer screening cohorts is unknown
- Purpose
- To determine whether a single visual emphysema score at LDCT is predictive of 25-year mortality from all causes, chronic obstructive pulmonary disease (COPD), and cardiovascular disease (CVD)
- Materials and Methods In this prospective cohort study, asymptomatic adults aged 40-85 years with a history of smoking underwent baseline LDCT screening for lung cancer between June 2000 and December 2008. Follow-up continued until death, loss to follow-up, or December 31, 2024. Emphysema was assessed at baseline LDCT and scored from 0 (none) to 3 (severe) by one of four experienced chest radiologists. Baseline smoking history and comorbidities were self-reported. Causes of death (International Classification of Diseases, 10th Revision) were obtained from the U.S. National Death Index, physicians, and family. Associations between emphysema and mortality were evaluated using adjusted Cox proportional hazards and adjusted Fine-Gray competing risks models
- Results
- Among 9047 participants (4614 female; median age, 65 years [IQR, 61-69 years]; median pack-years of smoking, 43 [IQR, 28-64]), 2637 (29.1%) had emphysema (mild in 1908 [21.1%], moderate in 512 [5.7%], and severe in 217 [2.4%]). Median follow-up was 23.3 years. Emphysema was independently predictive of all-cause mortality (hazard ratio [HR], 1.29; 95% CI: 1.21, 1.38; P < .001), COPD mortality (HR, 3.29; 95% CI: 2.59, 4.18; P < .001), and CVD mortality (HR, 1.14; 95% CI: 1.01, 1.29; P = .04). A dose-response relationship was observed between emphysema severity and both all-cause and COPD mortality, but not CVD mortality. In the adjusted competing risk analysis, emphysema remained associated with COPD mortality (HR, 3.06; 95% CI: 2.40, 3.90; P < .001), but not CVD mortality (HR, 1.04; 95% CI: 0.91, 1.18; P = .59)
- Conclusion
- Baseline emphysema at LDCT in a prospective lung cancer screening cohort of asymptomatic adults was predictive of all-cause, COPD, and CVD mortality up to 25 years later
- Background
Nocturnal Hypoxemia
- Epidemiology
- Nocturnal Hypoxemia
- Generally closely related to need for daytime oxygen: occurs in 27% of COPD patients with awake pO2 >60 mm Hg
- Normal subjects demonstrate small increases in pCO2 and small decreases in pO2 during sleep: these are accentuated in COPD patients
- Nocturnal desaturations in COPD are most marked in REM (probably due to decreased alveolar ventilation, inhibited respiratory muscles, decreased VT, decreased FRC with worsened V/Q mismatching during REM): typically worse in later REM periods
- Predictors of Nocturnal Hypoxemia in COPD
- Daytime pCO2 >45 mmg Hg
- Daytime pO2 <65 mm Hg
Chronic Hypoventilation (see Chronic Hypoventilation)
- CO2 retention in patients with COPD typically occurs when the FEV1 falls below 1L
- However, not all COPD patients with FEV1 <1L develop hypercapnia (due to multifactorial variability)
- Etiology of Acute Worsening of Pre-Existing Hypercapnia in COPD:
- Acute Pulmonary Embolism (PE)
- Pneumonia/Acute Bronchitis
- CHF
- Sedatives: such as antihistamines, benzodiazepines, etc.
- Zolpidem (which is an imidazopyridine) does not cause significant respiratory depression
- Mechanisms of Supplemental O2 Administration Worsening Pre-Existing Hypercapnia in COPD:
- Worsened V/Q Mismatch (Primary Etiology)
- Release of hypoxic vasoconstriction -> increase in blood flow to poorly ventilated lung segments -> increase in dead space and decrease in effective alveolar ventilation
- Release of Hypoxic Drive
- Decrease in VT + RR occurs transiently (usually for 15 min) and then increases back to pre-supplemental O2 baseline
- Haldane Effect
- Administration of oxygen causes concurrent decrease in the CO2 carrying capacity of the hemoglobin molecule -> resulting in a slight increase in pCO2
- Worsened V/Q Mismatch (Primary Etiology)
[Aubier M, Murciano D, Milic-Emili J, et al. Effects of the administration of O2 on the ventilation and blood gases in patients with chronic obstructive pulmonary disease during acute respiratory failure. Am Rev Respir Dis 1980; 122:747-754 Agusti A, Carrera M, Barbe F, et al. Oxygen therapy during exacerbations of chronic obstructive pulmonary disease. Eur Respir J 1999; 14:934-939 Murciano D, Armengaud M, Cramer P, et al. Acute effects of zolpidem, triazolam, flunitrazepam on arterial blood gases and control of breathing in severe COPD. Eur Respir J 1993; 6:625-629]
- Hypercapnia and lung function parameters in chronic obstructive pulmonary disease. BMC Pulm Med. 2024 Jul 16;24(1):345. doi: 10.1186/s12890-024-03151-1 [MEDLINE]
- Background
- In advanced chronic obstructive pulmonary disease (COPD), hypercapnia may occur due to severe bronchial obstruction with lung hyperinflation
- Non-invasive ventilation (NIV) provides the standard of care intended to achieve physiological PCO2 levels, thereby reducing overall mortality
- The present study aimed to evaluate pulmonary function parameters derived from spirometry (forced vital capacity [FVC], forced expiratory volume in 1 s [FEV1]), body plethysmography (residual volume [RV], total lung capacity [TLC]), and lung diffusion capacity for carbon monoxide (single-breath method [DCO-SB], alveolar-volume corrected values [DCO-VA]) as predictors of chronic hypercapnia in patients with advanced COPD
- Methods
- This monocentric, retrospective observational study included 423 COPD patients
- Receiver operating characteristic (ROC) curve analysis and cross-validation were used to assess lung function parameters’ diagnostic accuracy for predicting chronic hypercapnia, with the resulting performance expressed as area under the ROC curve (AUROC)
- We performed univariable and multivariable binary logistic regression analysis to determine if these parameters were independently associated with chronic hypercapnia, with probabilities reported as odds ratios [OR] with 95% confidence intervals [95%CI]
- Results: FVC% (AUROC 0.77 [95%CI 0.72-0.81], P < 0.01) and FEV1% (AURIC 0.75 [95%CI 0.70-0.79], P < 0.01) exhibited reasonable accuracy in the prediction of chronic hypercapnia, whereas lung diffusion capacity performed poorly (AUROC 0.64 [95%CI 0.58-0.71] for DCO-SB%, P < 0.01)
- FVC% (OR 0.95 [95%CI 0.93-0.97], P < 0.01) and FEV1% (OR 0.97 [95%CI 0.94-0.99], P = 0.029) were the only parameters associated independently with chronic hypercapnia in logistic regression analysis. FVC and FEV1 thresholds that best separated hypercapnic from normocapnic subjects reached 56% and 33% of predicted values
- Conclusions
- Routinely collected pulmonary function parameters, particularly FVC% and FEV1%, may predict chronic hypercapnia during COPD progression
- Background
Obstructive Sleep Apnea (OSA) (see Obstructive Sleep Apnea)
- Epidemiology
- Occurs in 10-15% of COPD patients
Pulmonary Hypertension/Cor Pulmonale (see Pulmonary Hypertension)
- Epidemiology
- In a Retrospective Study of 998 COPD Patients Who Underwent Swan-Ganz Catheterization, Only 1% had Severe Pulmonary Hypertension (PAP-Mean >40 mm Hg) (Am J Respir Crit Care Med, 2005) [MEDLINE]
- Study of the Prevalence of Pulmonary Hypertension in Advanced COPD Patients Referred for Lung Volume Reduction or Lung Transplant (Chest, 2005) [MEDLINE]
- Pulmonary Hypertension (PA-Mean >25 mm Hg) was Present in 50.2% of Patients
- Pulmonary Hypertension was Moderate (PA-Mean: 35-45 mm Hg) in 9.8% of Patients
- Pulmonary Hypertension was Severe (PA-Mean: >45 mm Hg) in 3.7% of Patients
- Study of Severe Pulmonary Hypertension in Patients with COPD (Chest, 2022) [MEDLINE]
- In patients with COPD, the combination of echocardiography, NT-proBNP level, and PA to Ao diameter ratio predicts severe PH with high sensitivity and specificity
- The contribution of severe PH and severe airflow limitation to impaired survival is comparable
- Physiology
- Due to chronic hypoxemia
- Clinical
- Symptoms of Right-Sided Congestive Heart Failure (Pulmonary Edema, etc) (see Congestive Heart Failure): variable
- Absence of Kussmaul Sign (see Kussmaul Sign)
- Prognosis
- Severe Pulmonary Hypertension in COPD: Impact on Survival and Diagnostic Approach. Chest. 2022 Jul;162(1):202-212. doi: 10.1016/j.chest.2022.01.031 [MEDLINE]
- Background: Severe pulmonary hypertension (PH) is prognostically highly relevant in patients with COPD. The criteria for severe PH have been defined based on hemodynamic thresholds in right heart catheterization
- Research question: Can noninvasive clinical tools predict severe PH in patients with COPD? How does the mortality risk change with increasing severity of airflow limitation and pulmonary vascular disease?
- Study design and methods: We retrospectively analyzed all consecutive patients with COPD with suspected PH undergoing in-depth clinical evaluation, including right heart catheterization, in our PH clinic between 2005 and 2018. Clinical variables potentially indicative of severe PH or death were analyzed using univariate and stepwise multivariate logistic regression and Cox regression analysis adjusted for age and sex
- Results: We included 142 patients with median FEV1 of 55.0% predicted (interquartile range [IQR], 42.4%-69.4% predicted) and mean pulmonary arterial pressure of 35 mm Hg (IQR, 27-43 mm Hg). A multivariate model combining echocardiographic systolic pulmonary arterial pressure of ≥ 56 mm Hg, N-terminal pro-brain natriuretic peptide (NT-proBNP) plasma levels of ≥ 650 pg/mL, and pulmonary artery (PA) to ascending aorta (Ao) diameter ratio on chest CT scan of ≥ 0.93 predicted severe PH with high positive and negative predictive values (both 94%). After correction for age and sex, both airflow limitation (P = .002; Global Initiative for Chronic Obstructive Lung Disease [GOLD] stages 1-2 vs stage 3: hazard ratio [HR], 1.56 [95% CI, 0.90-2.71]; GOLD stages 1-2 vs stage 4: HR, 3.45 [95% CI, 1.75-6.79]) and PH severity (P = .012; HR, 1.85 [95% CI, 1.15-2.99]) remained associated independently with survival. The combination of GOLD stages 3 and 4 airflow limitation and severe PH showed the poorest survival (HR for death, 3.26 [95% CI, 1.62-6.57; P = .001] vs GOLD stages 1-2 combined with nonsevere PH)
- Interpretation: In patients with COPD, the combination of echocardiography, NT-proBNP level, and PA to Ao diameter ratio predicts severe PH with high sensitivity and specificity. The contribution of severe PH and severe airflow limitation to impaired survival is comparable.
- Burden of pulmonary hypertension due to chronic obstructive pulmonary disease: Analysis of exacerbations and healthcare resource utilization in the United States. Respir Med. 2023 Sep 18:219:107412. doi: 10.1016/j.rmed.2023.107412 [MEDLINE]
- Background: The burden of pulmonary hypertension (PH) among patients with chronic obstructive pulmonary disease (COPD) is not well understood. The present retrospective cohort study aimed to quantify the clinical and economic burden of PH in patients with COPD
- Methods: Adults with COPD were retrospectively identified in the Optum® Clinformatics® Data Mart between July 1, 2016 and June 30, 2021. Those diagnosed with PH were assigned to the PH-COPD cohort and those without a diagnosis of PH were assigned to the COPD cohort. Outcomes, including the number of visits for exacerbations and all-cause and COPD-related healthcare resource utilization (HCRU) and costs per patient per month (PPPM), were compared between cohorts. Baseline and study outcomes were analyzed descriptively. For significance testing, continuous variables were analyzed using Student’s t-tests and categorical variables were analyzed using Chi-square tests
- Results: A total of 1627 patients with PH-COPD were matched 1:1 to COPD patients without PH. A greater percentage of PH-COPD patients experienced COPD exacerbations vs. the COPD cohort (p < 0.001) and the PH-COPD cohort had more total (p < 0.001) and severe exacerbation-related visits PPPM (p < 0.001). All-cause and COPD-related HCRU PPPM estimates were higher among the PH-COPD cohort vs. the COPD cohort (p < 0.01). Total all-cause (p < 0.001) and COPD-related costs (p < 0.001) were higher among PH-COPD patients than COPD patients
- Conclusions: Patients with PH-COPD had higher rates of severe exacerbations, hospitalizations, and costs compared to COPD patients without PH, underscoring the need for targeted therapies to prevent and manage PH in patients with COPD.
Other Manifestations
- Anorexia (see Anorexia)
- Epidemiology: common
- Cachexia: occurs in 20% of COPD outpatients, in 35% of pulm rehab candidates, and in 70% of COPD patients with acute respiratory failure
- Muscle wasting in COPD is associated with systemic inflammation (with low anabolic hormone levels and catabolism), rather than simple starvation and nutritional imbalance
- Cachexia iis associated with greater duration of mechanical ventilation after LVRS, lower exercise capacity, lower muscle strength, greater need for mechanical ventilation in acute exacerbation, impaired health status, increased rate of ealr nonelective readmission after an exacerbation, increased duration of ventilation post-lung transplant, increased hospital admission rates (in those on home oxygen), and worse survival rate
- Fatigue (see Fatigue)
- Epidemiology: common
Specific Clinical Features of Acute Chronic Obstructive Pulmonary Disease (COPD) Exacerbation

- Epidemiology
- Frequency of Chronic Obstructive Pulmonary Disease (COPD) Exacerbation Varies with Disease Severity (Global Initiative for Chronic Obstructive Lung Disease/GOLD. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease: 2026 Report) [LINK]
- UK Study of Chronic Obstructive Pulmonary Disease (COPD) Exacerbation Rate in a General Practice Population (Am J Respir Crit Care Med, 2018) [MEDLINE]: n = 99,574 patients with Chronic Obstructive Pulmonary Disease (COPD) (from January 1, 2004-March 31, 2015)
- Compared with no AECOPDs in the baseline period, AECOPD number predicted the future long-term rate of AECOPDs in a graduated fashion, ranging from hazard ratio (HR) of 1.71 (1.66-1.77) for one event to HR of 3.41 (3.27-3.56) for five or more events
- Two or more moderate AECOPDs were also associated with an increased risk of death in a graduated fashion, ranging from HR of 1.10 (1.03-1.18) for two moderate AECOPDs to HR of 1.57 (1.45-1.70) for five or more moderate AECOPDs, compared with those with no AECOPDs at baseline
- Severe AECOPDs were associated with an even higher risk of death (HR, 1.79; 1.65-1.94)
- Number of Chronic Obstructive Pulmonary Disease (COPD) Exacerbations in a baseline year of observation predicted the rate over the subsequent 10 years
- Approximately, 25% Did Not Experience an Exacerbation
- Those with one baseline exacerbation were likely to have another (hazard ratio [HR] 1.71, 95% CI 1.66-1.77)
- Those with ≥5 events were even more likely to have future events (HR 3.41, 95% CI 3.27-3.56)
- In a separate survey of more than 1000 patients, 21% of those who reported a COPD exacerbation required hospitalization (Respir Med, 2007) [MEDLINE]
- In a Survey that included over 4000 respondents with COPD, approximately 10-25% needed an emergency department evaluation for COPD and 5-10% required hospitalization (Int J Chron Obstruct Pulmon Dis, 2014) [MEDLINE]
- A Study of Medicare beneficiaries found a 64% readmission rate over one year following a discharge for COPD Exacerbation (Am J Respir Crit Care Med, 2018) [MEDLINE]
- Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1418 [MEDLINE]
- Patient’s perception of exacerbations of COPD–the PERCEIVE study. Respir Med. 2007;101(3):453 [MEDLINE]
- Continuing to Confront COPD International Patient Survey: methods, COPD prevalence, and disease burden in 2012-2013. Int J Chron Obstruct Pulmon Dis. 2014;9:597 [MEDLINE]
- Natural History of Chronic Obstructive Pulmonary Disease Exacerbations in a General Practice-based Population with Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2018;198(4):464 [MEDLINE]
- Risk Trajectories of Readmission and Death in the First Year after Hospitalization for Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2018;197(8):1009 [MEDLINE]
- Global Initiative for Chronic Obstructive Lung Disease/GOLD. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease: 2026 Report [LINK]
- Risk Factors for Chronic Obstructive Pulmonary Disease (COPD) Exacerbation
- Demographic Factors
- Advanced (Chronological) Age
- Biological Age
- Demographic Factors
- Modelling of biological age in stable and acute exacerbations of chronic obstructive pulmonary disease. BMC Pulm Med. 2025 Aug 19;25(1):398. doi: 10.1186/s12890-025-03841-4 [MEDLINE]
- Background: Aging has been established as an independent risk factor for chronic obstructive pulmonary disease (COPD). Biological age (BA), a novel metric for gauging the extent of aging, has rarely been investigated in the context of acute exacerbation of COPD (AECOPD). Our study aimed to elucidate the association between BA and AECOPD, thereby highlighting the potential of BA as a predictive tool in clinical practice
- Methods: The dataset encompasses patients hospitalized at Chengdu Third People’s Hospital between 2018 and 2022. The AECOPD patients enrolled in this study were hospitalized due to rapidly worsening symptoms, including cough, sputum production, and dyspnea, whereas the COPD patients were clinically stable. BA and biological age acceleration were ascertained through the Klemera-Doubale method (KDM). A multivariable logistic regression analysis was conducted to evaluate the correlation between BA, biological age acceleration, and the incidence of AECOPD, complemented by subgroup analyses to explore the dose‒response dynamics between biological age acceleration and the risk of AECOPD. The dataset was partitioned into training and validation sets at a 7:3 ratio, and LASSO regression was applied to refine the model’s variable composition. To assess the ability of different variables to discriminate current disease status, we developed the initial model and three subsequent models, with the following variables added in the new model: Chronological age (CA), BA, and biological age acceleration. The models were subsequently evaluated within both datasets
- Results: The study cohort comprised 2,511 patients, through an analysis of the transect data, with 59.1% experiencing acute exacerbations. Both BA (79.14 ± 9.49 years) and biological age acceleration (1.04 ± 2.82 years) emerged as independent risk factors for AECOPD (P < 0.001). In Model 3, each year increment in BA and biological age acceleration corresponded to a 1.04-fold (95% CI = 1.027-1.048, P < 0.001) and 1.18-fold (95% CI = 1.14-1.224, P < 0.001) increase in exacerbation risk, respectively. The biological age of patients with stable COPD was significantly lower than the actual age (-0.36 ± 2.56 years), which suggests a significant inter-individual heterogeneity in the biological aging process of COPD patients. Subgroup analysis confirmed a pronounced dose‒response relationship between biological age acceleration and AECOPD risk(Q4 vs. Q1: OR = 2.7, 95% CI = 2.172-3.518). LASSO regression pinpointed BMI, Diabetes, Hypertensive heart disease, Cor pulmonale, Stroke, and Hyperlipidemia as critical variables within the model. The internal validation process revealed AUC values of 0.735 (95% CI = 0.7-0.77), 0.742 (95% CI = 0.707-0.777), 0.753 (95% CI = 0.719-0.787), and 0.766 (95% CI = 0.733-0.8) for the respective models. The HL test confirmed the models’ good fit (P = 0.128, P = 0.121, P = 0.272, P = 0.795), with Model 4 exhibiting the most precise calibration against the diagonal reference. Decision curve analysis (DCA) indicated that all the models provided a net benefit in disease outcome discrimination, with Model 4 yielding the most significant advantage
- Conclusions: The acceleration of aging portends an increased propensity for acute exacerbations, and a distinct dose-response relationship is observable between biological age acceleration and exacerbation events. BA and biological age acceleration outperform chronological age in discerning the likelihood of acute exacerbations, underscoring their enhanced ability to predict this critical health outcome.
- Disease-Related Factors
- History of Antibiotic Treatment for Respiratory Disease
- History of Chronic Obstructive Pulmonary Disease (COPD) Exacerbations
- History of Chronic Obstructive Pulmonary Disease (COPD)-Related Hospitalization in the Prior One Year
- Chronic Mucous Hypersecretion/Plugs
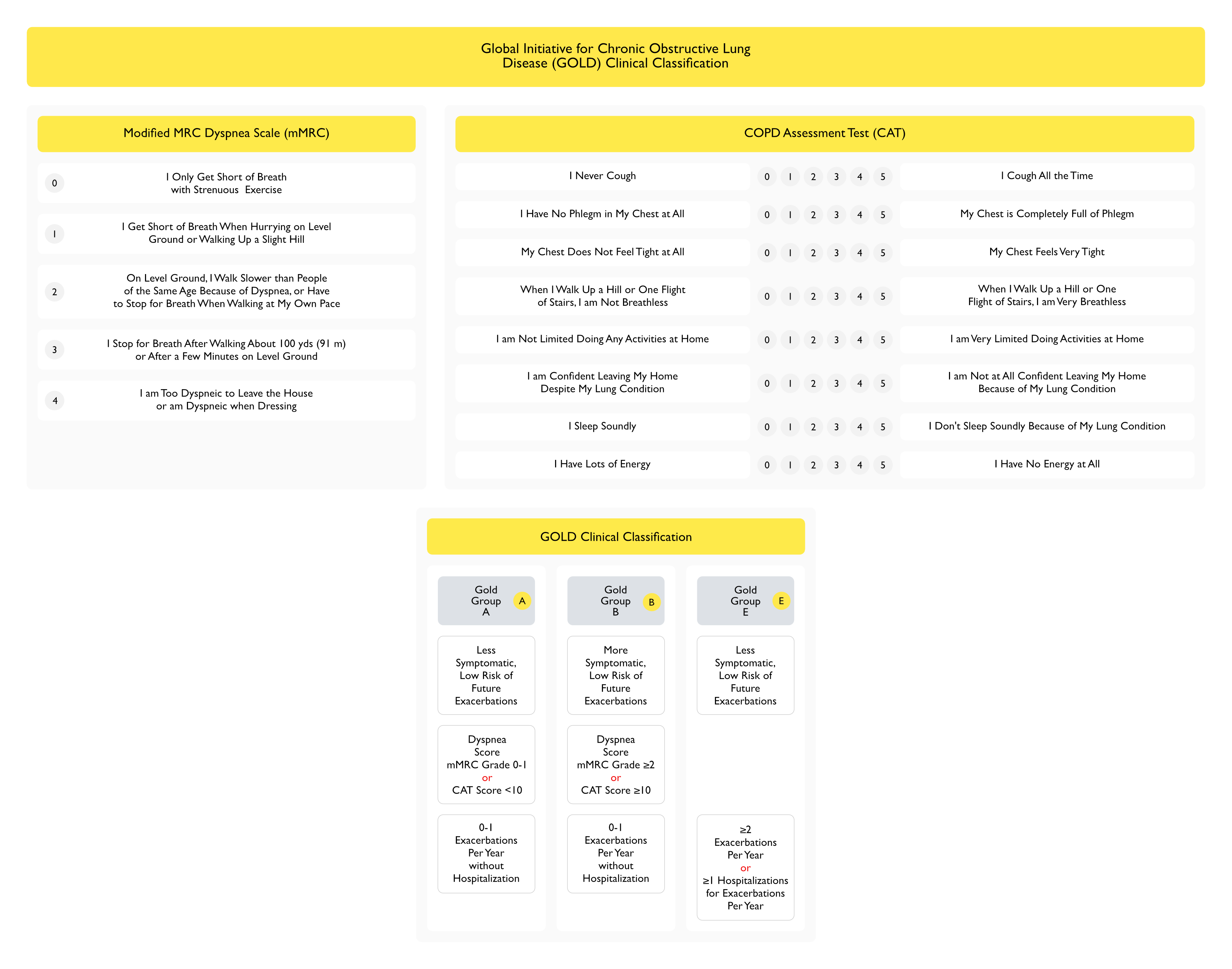
- Dyspnea Scale Score mMRC ≥2 or CAT >10
- Longer Duration of Chronic Obstructive Pulmonary Disease (COPD)
- Peripheral Eosinophilia (≥340 Cells/μL) (see Peripheral Eosinophilia)
- Productive Cough (see Cough)
- Severe Airflow Obstruction (FEV1 <30% Predicted)
- In General, Worsening Airflow Limitation (Decreased FEV1) is Associated with an Increased Risk of Chronic Obstructive Pulmonary Disease (COPD) Exacerbation, Although Airflow Limitation Alone Does Not Provide a Good Assessment of Exacerbation Risk
- Rate of Chronic Obstructive Pulmonary Disease (COPD) Exacerbations is Related to the GOLD Spirometric Class (GOLD; Global Strategy for Diagnosis, Management, and Prevention of COPD, 2016) [LINK]
- GOLD 1 (Mild, FEV1 ≥80% Predicted): unknown annual exacerbation rate
- GOLD 2 (Moderate, FEV1 50-80% Predicted): 0.7-0.9 annual exacerbation rate
- GOLD 3 (Severe, FEV1 30-50% Predicted): 1.1-1.3 annual exacerbation rate
- GOLD 4 (Very Severe, FEV1 <30% Predicted): 1.2-2.0 annual exacerbation rate
- Theophylline Use (see Theophylline)
- Comorbid Disease
- Asthma-COPD Overlap (ACO) (see Asthma)
- Congestive Heart Failure (CHF) with Left Ventricular Diastolic Dysfunction (see Congestive Heart Failure)
- Coronary Artery Disease (CAD) (see Coronary Artery Disease)
- Decreased Serum IgG
- Lower levels of serum IgG may be associated with an increased risk for COPD exacerbations and hospitalization [35,36]
- In one prospective cohort study, patients in the lowest tertile of serum IgG concentration (less than 1225 mg/dL) had a higher exacerbation risk compared with the rest of the cohort (incidence rate ratio [IRR] 1.28, 95% CI 1.08–1.51), even after accounting for demographics, smoking history, and COPD severity [36]. Among IgG subclasses, IgG1 and IgG2 were associated with an increased risk of severe exacerbation (IRR 1.39, 95% CI 1.06–1.84; and IRR 1.50, 95% CI 1.14–1.97)
- Diabetes Mellitus (see Diabetes Mellitus)
- Gastroesophageal Reflux Disease (GERD) (see Gastroesophageal Reflux Disease)
- Pulmonary Hypertension (see Pulmonary Hypertension)
- Medications Causing Sedation/Respiratory Depression
- Benzodiazepines (see Benzodiazepines)
- Gabapentinoids (Gabapentin, Pregabalin) (see Gabapentin or Pregabalin)
- Opioids (see Opioids)
- Triggers of Chronic Obstructive Pulmonary Disease (COPD) Exacerbation (NEJM, 2000)[MEDLINE] (Thorax, 2006) [MEDLINE]
- Respiratory Infection (Account for 70% of Chronic Obstructive Pulmonary Disease/COPD Exacerbations)
- Bacterial Infection
- General Comments
- XXXX
- Enterobacteriaceae Have Been Isolated from the Respiratory Tract of 3-19% of Patients with Chronic Obstructive Pulmonary Disease (COPD) Exacerbations, But Their Pathogenic Significance in this Setting is Unclear
- Staphylococcus Aureus Has Been Isolated from the respiratory tract of 1-20% of patients with Chronic Obstructive Pulmonary Disease (COPD) Exacerbations, But Their Pathogenic Significance in this Setting is Unclear
- Haemophilus Parainfluenzae Has Been Isolated from the respiratory tract of 2-32% of Patients with Chronic Obstructive Pulmonary Disease (COPD) Exacerbations, But This Organism is Unlikely to Cause Chronic Obstructive Pulmonary Disease (COPD) Exacerbation
- Atypical Bacteria are a Relatively Uncommon Etiology of Chronic Obstructive Pulmonary Disease (COPD) Exacerbations (NEJM, 2008) [MEDLINE] (Respirology, 2010) [MEDLINE]
- Haemophilus Influenzae (see Haemophilus Influenzae)
- Accounts for 13-50% of Bacterial Pathogens in Chronic Obstructive Pulmonary Disease (COPD) Exacerbations (Proc Am Thorac Soc, 2004) [MEDLINE]
- Moraxella Catarrhalis (see Moraxella Catarrhalis)
- Accounts for 9-21% of Bacterial Pathogens in Chronic Obstructive Pulmonary Disease (COPD) Exacerbations (Proc Am Thorac Soc, 2004) [MEDLINE]
- Streptococcus Pneumonia (see Streptococcus Pneumonia)
- Accounts for 7-26% of Bacterial Pathogens in Chronic Obstructive Pulmonary Disease (COPD) Exacerbations (Proc Am Thorac Soc, 2004) [MEDLINE]
- Pseudomonas Aeruginosa (see Pseudomonas Aeruginosa)
- Accounts for 1-13% of Bacterial Pathogens in Chronic Obstructive Pulmonary Disease (COPD) Exacerbations (Proc Am Thorac Soc, 2004) [MEDLINE]
- Viral Infection
- Human Metapneumovirus (see Human Metapneumovirus)
- Influenza Virus (see Influenza)
- Parainfluenza Virus (see Parainfluenza Virus)
- Respiratory Syncytial Virus (RSV) (see Respiratory Syncytial Virus)
- Rhinovirus (see Rhinovirus)
- Fungal Infection
- Aspergillus (see Aspergillus): although Aspergillus may be identified from sputum during moderate-severe exacerbations, its clinical relevance (with respect to triggering the exacerbation) is unclear
- Bacterial Infection
- Indoor/Outdoor Air Pollutants (Am J Respir Crit Care Med, 2013) [MEDLINE] (PLoS One, 2019) [MEDLINE] (Thorax, 2023) [MEDLINE] (Thorax, 2024) [MEDLINE]
- Carbon Monoxide (see Carbon Monoxide)
- Inhalable Particulate Matter (≤10 Microns, aka PM10)
- Increased Ambient Levels of Fine Particulate Matter (≤2.5 Microns, aka PM2.5) are Associated with an Increase in Hospitalization and Mortality in Chronic Obstructive Pulmonary Disease (COPD) (Chest, 2016) [MEDLINE]
- In a trial of 116 patients with COPD and exposure to poor air quality at home (PM2.5 >10 mcg/m3), use of indoor air filters versus sham air filters resulted in a dose-dependent improvement in respiratory symptoms and risk of moderate exacerbations (Am J Respir Crit Care Med, 2022) [MEDLINE]
- Nitrogen Dioxide (see Nitrogen Dioxide)
- Ozone (see Ozone)
- Indoor/Outdoor Temperature Conditions
- Low Outdoor Temperatures are Associated with an Increased Risk of Chronic Obstructive Pulmonary Disease (COPD) Exacerbations (PLoS One, 2013) [MEDLINE] (Environ Health Perspect, 2014) [MEDLINE]
- High Outdoor Temperatures are Associated with an Increased Risk of Hospitalization for Chronic Obstructive Pulmonary Disease (COPD) (PLoS One, 2013) [MEDLINE] (Environ Health Perspect, 2014) [MEDLINE]
- Indoor Temperature/Humidity Can Vary Based on Building Type/Insulation/Socioeconomic Factors/Behavioral Factors, and Have Been Associated with Increased Dyspnea and Short-Acting Bronchodilator Use (Int J Biometeorol, 2017) [MEDLINE] (ERJ Open Res, 2022) [MEDLINE]
- Other Medical Conditions
- Acute Pulmonary Embolism (PE) (Ann Intern Med, 2006) [MEDLINE]
- The Frequency of Acute Pulmonary Embolism (PE) Among Patients Hospitalized for Chronic Obstructive Pulmonary Disease (COPD) Exacerbation Varies Across Studies (J Emerg Med, 2018) [MEDLINE] (Respir Care, 2019) [MEDLINE] (JAMA, 2021) [MEDLINE] (JAMA, 2021) [MEDLINE]
- Systematic Review of Patients Presenting with Chronic Obstructive Pulmonary Disease (COPD) Exacerbation Who were Evaluated by CT Pulmonary Artery Angiogram within 48 hrs of Hospital Admission (Am J Emerg Med, 2021) [MEDLINE]: n = 999 (from 7 studies)
- Pooled Prevalence of Acute Pulmonary Embolism was 23%
- Prospective Multicenter Study of Patients who were Admitted to the Hospital with Acute Worsening of Respiratory Symptoms with All Patients Being Screened with CT Pulmonary Artery Angiogram (Int J Chron Obstruct Pulmon Dis, 2023) [MEDLINE]: n = 1580 Patients with Chronic Obstructive Pulmonary Disease (COPD)
- Pulmonary Embolism was Found in 17% (11% Had Acute Pulmonary Embolism Involving the Main/Lobar Pulmonary Arteries)
- The Presence of New Purulent Sputum Decreased the Odds of Diagnosing Acute Pulmonary Embolism (PE)
- An Important Limitation of These Studies is Their Inability to Determine Whether the Acute Pulmonary Embolism (PE) is the Etiology of the Chronic Obstructive Pulmonary Disease (COPD) Exacerbation, a Result of the Chronic Obstructive Pulmonary Disease (COPD) Exacerbation, or a Mere Bystander
- Aspiration (see Aspiration Pneumonia)
- Congestive Heart Failure (CHF) (see Congestive Heart Failure)
- Myocardial Ischemia (see Coronary Artery Disease)
- Acute Pulmonary Embolism (PE) (Ann Intern Med, 2006) [MEDLINE]
- Respiratory Infection (Account for 70% of Chronic Obstructive Pulmonary Disease/COPD Exacerbations)
- Low human beta-defensin-2 levels in the sputum of COPD patients are associated with the risk of exacerbations. BMC Pulm Med. 2023 Mar 31;23(1):106. doi: 10.1186/s12890-023-02364-0 [MEDLINE]
- Rationale: Chronic obstructive pulmonary disease (COPD) is a complicated chronic inflammatory disease. It is important to investigate the characteristics of acute exacerbation of COPD to develop new therapeutic strategies.
- Objective: This study aimed to determine the relationship between the human beta-defensin-2 (hBD-2) levels and aggravation of COPD.
- Methods: We detected the sputum hBD-2 level of 254 patients from Guangzhou, China, for 2 years. The study participants were categorized into the COPD group (n = 203, GOLD 0-4) and the control group (n = 51, 40-79 years old). At baseline, 12th month, and 24th month, we detected the sputum hBD-2 level and levels of cytokines, such as CXCL10, CXCL11, and IFN
- Results: At baseline, there were no significant differences in the sputum and serum hBD-2 levels between the patients and the controls. However, the sputum hBD-2 levels of patients who had at least one symptom aggravation over the next 2 years were significantly lower than those of patients without any exacerbations (1130.9 ± 858.4 pg/mL vs. 2103.7 ± 1294.2 pg/mL, respectively; p = 0.001). Nevertheless, there were no statistically significant differences in the sputum hBD-2 levels between patients (no aggravation history) and controls (2084.9 ± 1317.6 pg/mL vs. 2152.5 ± 1251.6 pg/mL, respectively; p = 0.626). We used a logistic regression model to assess the relationship between aggravation and sputum hBD-2 levels. Interestingly, we found that low hBD-2 level (< 1000 pg/mL) was significantly associated with exacerbations. Specifically, patients with low hBD-2 levels were more likely to experience exacerbations in the next 12 months (0.333 vs. 0.117; p = 0.001). Moreover, we compared the hBD-2 levels between controls and patients with GOLD 3-4 and found that participants with bacteria (+) and/or viruses (+) had an association between hBD-2 level and disease severity (p = 0.02)
- Conclusion: Patients at risk of exacerbations are more likely to have lower sputum hBD-2 levels. These results have important implications for future therapies for COPD.
- Differential Association of COPD Subtypes With Cardiovascular Events and COPD Exacerbations. Chest. 2024 Dec;166(6):1360-1370. doi: 10.1016/j.chest.2024.07.148 [MEDLINE]
- Background: The coronary artery calcium score (CACS) and ratio of the pulmonary artery to aorta diameters (PA:A ratio) measured from chest CT scans have been established as predictors of cardiovascular events and COPD exacerbations, respectively. However, little is known about the reciprocal relationship between these predictors and outcomes. Furthermore, the prognostic implications of COPD subtypes on clinical outcomes remain insufficiently characterized
- Research question: How can these two chest CT scan-derived parameters predict subsequent cardiovascular events and COPD exacerbations in different COPD subtypes?
- Study design and methods: Using COPDGene study data, we assessed prospective cardiovascular disease (CVD) and COPD exacerbation risk in participants with COPD (Global Initiative for Chronic Obstructive Lung Disease spirometric grades 2-4), focusing on CACS and PA:A ratio at study enrollment, with logistic regression models. These outcomes were analyzed in three COPD subtypes: 1,042 participants with non-emphysema-predominant COPD (NEPD; low attenuation area at -950 Hounsfield units [LAA-950] < 5%), 1,324 participants with emphysema-predominant COPD (EPD; LAA-950 ≥ 10%), and 465 participants with intermediate emphysema COPD (IE; 5% ≤ LAA-950 < 10%)
- Results: Our study indicated significantly higher overall risk for cardiovascular events in participants with higher CACS (≥ median; OR, 1.61; 95% CI, 1.30-2.00) and increased COPD exacerbations in those with higher PA:A ratios (≥ 1; OR, 1.80; 95% CI, 1.46-2.23). Notably, participants with NEPD showed a stronger association between these indicators and clinical events compared to EPD (with CACS/CVD, NEPD vs EPD: OR, 2.02 vs 1.41; with PA:A ratio/COPD exacerbation, NEPD vs EPD: OR, 2.50 vs 1.65); the difference in ORs between COPD subtypes was statistically significant for CACS/CVD
- Interpretation: Two chest CT scan parameters, CACS and PA:A ratio, hold distinct predictive values for cardiovascular events and COPD exacerbations that are influenced by specific COPD subtypes.
- Clinical Features
- Acute Hypoxemic Respiratory Failure or Acute Hypoxemic, Hypercapnic Respiratory Failure (see Respiratory Failure)
- Dyspnea (see Dyspnea)
- Due to Increased Airway/Systemic Inflammation, Hyperinflation, Gas Trapping, Decreased Expiratory Airflow Rates, and/or V/Q Mismatch
- Increased Serum Troponin (see Increased Serum Troponin)
- Tachycardia (see Sinus Tachycardia)
- Tachypnea (see Tachypnea)
- Wheezing (see Wheezing)
- Rome Classification of Chronic Obstructive Pulmonary Disease (COPD) Exacerbation Severity (Am J Respir Crit Care Med, 2021) [MEDLINE] (Global Initiative for Chronic Obstructive Lung Disease/GOLD. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease: 2026 Report) [LINK]
- Mild: usually treated with short-acting bronchodilators
- Dyspnea Visual Analog Scale <5
- Respiratory Rate <24 breaths/min
- Heart Rate <95 beats/min
- Resting SpO2 ≥92% on Room Air (or on Patient’s Usual Oxygen Prescription) and Change ≤3% from Baseline
- CRP <10 mg/L
- Moderate (Any 3 of the Following): usually treated with short-acting bronchodilators + antibiotics +/- oral glucocorticoids
- Dyspnea Visual Analog Scale ≥5
- Respiratory Rate ≥24 breaths/min
- Heart Rate ≥95 beats/min
- Resting SpO2 <92% on Room Air (or on Patient’s Usual Oxygen Prescription) and/or Change >3% from Baseline
- CRP ≥10 mg/L
- Severe: may be associated with respiratory failure and require noninvasive positive-pressure ventilation (NIPPV)/invasive mechanical ventilation
- Dyspnea Visual Analog Scale ≥5
- Respiratory Rate ≥24 breaths/min
- Heart Rate ≥95 beats/min
- Resting SpO2 <92% on Room Air (or on Patient’s Usual Oxygen Prescription) and/or Change >3% from Baseline
- CRP ≥10 mg/L
- Arterial Blood Gas (ABG) with Hypoxemia (pO2 ≤60 mm Hg) and/or Hypercapnia (pCO2 >45 mm Hg and pH <7.35)
- Mild: usually treated with short-acting bronchodilators
- Inpatient Classification of Chronic Obstructive Pulmonary Disease (COPD) Exacerbation Severity (Am J Respir Crit Care Med, 2021) [MEDLINE]
- No Respiratory Failure
- Respiratory Rate (RR) ≤24 breaths/min
- Heart Rate (HR) <95 beats/min
- Absence of Accessory Respiratory Muscle Use
- Absence of Altered Mental Status
- SpO2 88-92% on Venturi Mask with 24-35% Inspired Oxygen (or Equivalent)
- Absence of Hypercapnia
- Acute Non-Life-Threatening Respiratory Failure
- Respiratory Rate (RR) >24 breaths/min
- Accessory Respiratory Muscle Use
- Absence of Altered Mental Status
- SpO2 88-92% on Venturi Mask with 24-35% Inspired Oxygen (or Equivalent)
- pCO2 50-60 mm Hg (or Increased Over Baseline)
- Acute Life-Threatening Respiratory Failure
- Respiratory Rate (RR) >24 breaths/min
- Accessory Respiratory Muscle Use
- Altered Mental Status
- Requiring FiO2 ≥40% to Maintain SpO2 88-92%
- pCO2 or >60 mm Hg (or pCO2 Increased Over Baseline and Associated with Acidosis, pH ≤7.25)
- No Respiratory Failure
- Interventions Which Decrease the Risk of Future Chronic Obstructive Pulmonary Disease (COPD) Exacerbations (Global Initiative for Chronic Obstructive Lung Disease/GOLD. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease: 2026 Report) [LINK]
- Bronchodilator Inhaled Maintenance Therapy
- LABA
- LAMA
- LABA + LAMA
- Corticosteroid-Containing Bronchodilator Inhaled Maintenance Therapy
- LABA + LAMA + Inhaled Corticosteroid
- Anti-Inflammatory
- Dupilumab (Dupixent) (see Dupilumab)
- Mepolizumab (Nucala) (see Mepolizumab)
- Roflumilast (Daliresp, Daxas) (see Roflumilast)
- Anti-Infective Measures
- Long-Term Macrolides (see Azithromycin)
- Vaccination
- Influenza Virus (see Influenza)
- Respiratory Syncytial Virus (RSV) (see Respiratory Syncytial Virus)
- Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV2) (see Severe Acute Respiratory Syndrome Coronavirus-2)
- Streptococcus Pneumoniae (see Streptococcus Pneumoniae)
- Mucoregulators
- Carbocysteine (see Carbocysteine)
- Erdosteine
- N-Acetylcysteine (see N-Acetylcysteine)
- Others
- Glucagon-Like Peptide-1 (GLP-1) Receptor Agonists/Sodium-Glucose Cotransporter-2 (SGLT-2) Inhibitors (see Glucagon-Like Peptide-1 Receptor Agonists and Sodium-Glucose Cotransporter-2 Inhibitors)
- Decrease Frequency of Chronic Obstructive Pulmonary Disease (COPD) Exacerbations in Diabetic Patients
- Lung Volume Reduction (see xxxx)
- Noninvasive Positive-Pressure Ventilation (NIPPV) (see Noninvasive Positive-Pressure Ventilation)
- In Patients Who Require Noninvasive Positive-Pressure Ventilation (NIPPV) During a Hospitalization for a Chronic Obstructive Pulmonary Disease (COPD) Exacerbation and Who Remain Hypercapnic, Nocturnal Noninvasive Positive-Pressure Ventilation (NIPPV) at Home Significantly Decreases the Risk of Rehospitalization
- Physical Activity
- Pulmonary Rehabilitation (see Pulmonary Rehabilitation)
- Shielding Measures
- Frequent Hand Washing
- Masks
- Minimization of Social Contact
- Smoking Cessation (see Tobacco)
- Vitamin D (see Vitamin D)
- Decreases Frequency of Chronic Obstructive Pulmonary Disease (COPD) Exacerbations in Patients with Severe Vitamin D Deficiency
- Glucagon-Like Peptide-1 (GLP-1) Receptor Agonists/Sodium-Glucose Cotransporter-2 (SGLT-2) Inhibitors (see Glucagon-Like Peptide-1 Receptor Agonists and Sodium-Glucose Cotransporter-2 Inhibitors)
- Bronchodilator Inhaled Maintenance Therapy
Prevention of Acute Exacerbations of Chronic Obstructive Pulmonary Disease (COPD)
- Recommendations (American College of Chest Physicians/Canadian Thoracic Society Guidelines on the Prevention of Chronic Obstructive Pulmonary Disease (COPD) Exacerbations, 2015) (Chest, 2015) [MEDLINE]
- Vaccination
- 23-Valent Pneumococcal Vaccination is Recommended, Although There is No Evidence that it Decreases Chronic Obstructive Pulmonary Disease (COPD) Exacerbation Rate (Grade 2C Recommendation)
- Annual Influenza Vaccination is Recommended to Decrease the Chronic Obstructive Pulmonary Disease (COPD) Exacerbation Rate (Grade 1B Recommendation)
- Smoking Cessation is Recommended to Decrease the Chronic Obstructive Pulmonary Disease (COPD) Exacerbation Rate (Grade 2C Recommendation) (see Tobacco)
- Pulmonary Rehabilitation (see Pulmonary Rehabilitation)
- In Moderate-Very Severe Chronic Obstructive Pulmonary Disease (COPD) with a Recent Exacerbation (Within 4 wks), Pulmonary Rehabilitation is Recommended to Decrease the Chronic Obstructive Pulmonary Disease (COPD) Exacerbation Rate (Grade 1C Recommendation)
- In Moderate-Very Severe Chronic Obstructive Pulmonary Disease (COPD) with an Exacerbation >4 wks Ago, Pulmonary Rehabilitation is Not Recommended to Decrease the Chronic Obstructive Pulmonary Disease (COPD) Exacerbation Rate (Grade 2B Recommendation)
- Education, Case Management, and Action Plan
- Education Alone is Not Recommended to Decrease the Chronic Obstructive Pulmonary Disease (COPD) Exacerbation Rate (Ungraded Recommendation)
- Case Management Alone is Not Recommended to Decrease the Chronic Obstructive Pulmonary Disease (COPD) Exacerbation Rate (Ungraded Recommendation)
- In Patients with a History of Prior or Recent Exacerbations, Education and Case Management (with Access to a Healthcare Provider at Least Monthly) is Recommended to Decrease the Severe Chronic Obstructive Pulmonary Disease (COPD) Exacerbation Rate (as Assessed by the Hospitalization Rate) (Grade 1C Recommendation)
- In Patients with Moderate-Severe Chronic Obstructive Pulmonary Disease (COPD), Education with an Action Plan (But Without Case Management) is Not Recommended to Decrease the Severe Chronic Obstructive Pulmonary Disease (COPD) Exacerbation Rate (as Assessed by a Decrease in Emergency Department Visits or Hospitalizations Over a 12 mo Period) (Grade 2C Recommendation)
- Education with a Written Action Plan and Case Management is Recommended for the Prevention of Severe Acute Chronic Obstructive Pulmonary Disease (COPD) Exacerbations (as Assessed by a Decrease in Emergency Department Visits or Hospitalizations) (Grade 2B)
- Telemonitoring
- Telemonitoring (as Compared to Usual Care) is Not Recommended to Decrease the Chronic Obstructive Pulmonary Disease (COPD) Exacerbation Rate (as Assessed by Decreases in Emergency Room Visits, Exacerbations, or Hospitalizations Over a 12 mo Period (Grade 2C Recommendation)
- Inhaled Maintenance Therapy
- In Moderate-Severe Chronic Obstructive Pulmonary Disease (COPD), Long-Acting β2-Agonists (as Compared to Placebo) are Recommended to Prevent Moderate-Severe Acute Chronic Obstructive Pulmonary Disease (COPD) Exacerbations (Grade 1B (Grade 1B Recommendation)
- In Moderate-Severe Chronic Obstructive Pulmonary Disease (COPD), Long-Acting Muscarinic Antagonists (as Compared to Placebo) are Recommended to Prevent Moderate-Severe Acute Chronic Obstructive Pulmonary Disease (COPD) Exacerbations (Grade 1A Recommendation)
- In Moderate-Severe Chronic Obstructive Pulmonary Disease (COPD), Long-Acting Muscarinic Antagonists (as Compared to Long-Acting β2-Agonists) are Recommended to Prevent Moderate-Severe Acute Chronic Obstructive Pulmonary Disease (COPD) Exacerbations (Grade 1C Recommendation)
- In Moderate-Severe Chronic Obstructive Pulmonary Disease (COPD), Short-Acting Muscarinic Antagonist Monotherapy (as Compared to Short-Acting β2-Agonist Monotherapy) is Recommended to Prevent Mild-Moderate Acute COPD Exacerbations (Grade 2C Recommendation)
- In Moderate-Severe Chronic Obstructive Pulmonary Disease (COPD), Short-Acting Muscarinic Antagonist + Short-Acting β2-Agonist Combination Therapy (as Compared to Short-Acting β2-Agonist Monotherapy) is Recommended to Prevent Moderate Acute Chronic Obstructive Pulmonary Disease (COPD) Exacerbations (Grade 2B Recommendation)
- In Moderate-Severe Chronic Obstructive Pulmonary Disease (COPD), Long-Acting β2-Agonist Monotherapy (as Compared to Short-Acting Muscarinic Antagonist Monotherapy) is Recommended to Prevent Acute Chronic Obstructive Pulmonary Disease (COPD) Exacerbations (Grade 2C Recommendation)
- In Moderate-Severe Chronic Obstructive Pulmonary Disease (COPD), Long-Acting Muscarinic Antagonist Monotherapy (as Compared to Short-Acting Muscarinic Antagonist Monotherapy) is Recommended to Prevent Moderate-Severe Acute Chronic Obstructive Pulmonary Disease (COPD) Exacerbations (Grade 1A Recommendation)
- In Moderate-Severe Chronic Obstructive Pulmonary Disease (COPD), Short-Acting Muscarinic Antagonist + Long-Acting β2-Agonist Combination Therapy (as Compared to Long-Acting β2-Agonist Monotherapy) is Recommended to Prevent Mild-Moderate Acute COPD Exacerbations (Grade 2C Recommendation)
- In Stable Moderate-Very Severe Chronic Obstructive Pulmonary Disease (COPD), Inhaled Corticosteroid + Long-Acting β2-Agonist Combination Therapy (as Compared to Placebo) is Recommended to Prevent Acute Chronic Obstructive Pulmonary Disease (COPD) Exacerbations (Grade 1B Recommendation)
- In Stable Moderate-Very Severe Chronic Obstructive Pulmonary Disease (COPD), Inhaled Corticosteroid + Long-Acting β2-Agonist Combination Therapy (as Compared to Long-Acting β2-Agonist Monotherapy) is Recommended to Prevent Acute Chronic Obstructive Pulmonary Disease (COPD) Exacerbations (Grade 1C Recommendation)
- In Stable Moderate-Very Severe Chronic Obstructive Pulmonary Disease (COPD), Inhaled Corticosteroid + Long-Acting β2-Agonist Combination Therapy (as Compared to Inhaled Corticosteroid Monotherapy) is Recommended to Prevent Acute Chronic Obstructive Pulmonary Disease (COPD) Exacerbations (Grade 1B Recommendation)
- In Stable Chronic Obstructive Pulmonary Disease (COPD), Long-Acting Muscarinic Antagonist + Long-Acting β2-Agonist Combination Therapy or Long-Acting Muscarinic Antagonist Monotherapy are Both Recommended to Prevent Acute Chronic Obstructive Pulmonary Disease (COPD) Exacerbations (Grade 1C Recommendation): both are equally effective
- In Stable Chronic Obstructive Pulmonary Disease (COPD), Inhaled Corticosteroid + Long-Acting β2-Agonist Combination Therapy or Long-Acting Muscarinic Antagonist Monotherapy are Both Recommended to Prevent Acute COPD Exacerbations (Grade 1C Recommendation): both are equally effective
- In Stable Chronic Obstructive Pulmonary Disease (COPD), Inhaled Corticosteroid + Long-Acting Muscarinic Antagonist + Long-Acting β2-Agonist Triple Therapy or Long-Acting Muscarinic Antagonist Monotherapy are Both Recommended to Prevent Acute Chronic Obstructive Pulmonary Disease (COPD) Exacerbations (Grade 2C Recommendation): both are equally effective
- Macrolides (Azithromycin, etc) (see Macrolides)
- In Moderate-Severe Chronic Obstructive Pulmonary Disease (COPD) with History of ≥1 Moderate-Severe Chronic Obstructive Pulmonary Disease (COPD) Exacerbations in Prior Year Despite Optimal Maintenance Inhaler Therapy, Long-Term Macrolide is Recommended to Decrease the Chronic Obstructive Pulmonary Disease (COPD) Exacerbation Rate (Grade 2A Recommendation)
- Systemic Corticosteroids (see Corticosteroids)
- For Patients with Acute Chronic Obstructive Pulmonary Disease (COPD) Exacerbation in Inpatient/Outpatient Setting, Systemic Corticosteroids (Orally or Intravenously) are Recommended to Prevent Hospitalization for Subsequent Acute Chronic Obstructive Pulmonary Disease (COPD) Exacerbation in the the Next 30 Days Following the Initial Exacerbation (Grade 2B Recommendation)
- For Patients with Acute Chronic Obstructive Pulmonary Disease (COPD) Exacerbation in Inpatient/Outpatient Setting, Systemic Corticosteroids (Orally or Intravenously) are Not Recommended For the Sole Purpose of Preventing Hospitalization for Subsequent Acute COPD Exacerbation Beyond the First 30 Days Following the Initial Exacerbation (Grade 1A Recommendation)
- Roflumilast (Daliresp) (see Roflumilast)
- In Moderate-Severe Chronic Obstructive Pulmonary Disease (COPD) with Chronic Bronchitis and a History of ≥1 Chronic Obstructive Pulmonary Disease (COPD) Exacerbation in the Prior Year, Roflumilast is Recommended to Decrease the Chronic Obstructive Pulmonary Disease (COPD) Exacerbation Rate (Grade 2A Recommendation)
- Theophylline (see Theophylline)
- In Stable Chronic Obstructive Pulmonary Disease (COPD), Oral Slow-Release Theophylline BID is Recommended to Decrease the Chronic Obstructive Pulmonary Disease (COPD) Exacerbation Rate (Grade 2B Recommendation)
- N-Acetylcysteine (Mucomyst) (see N-Acetylcysteine)
- In Moderate-Severe Chronic Obstructive Pulmonary Disease (COPD) and a History of ≥2 Chronic Obstructive Pulmonary Disease (COPD) Exacerbations in the Prior 2 Years, Oral N-Acetylcysteine is Recommended to Decrease the Chronic Obstructive Pulmonary Disease (COPD) Exacerbation Rate (Grade 2B Recommendation)
- Carbocysteine (see Carbocysteine)
- In Stable Outpatient Chronic Obstructive Pulmonary Disease (COPD) with Continued Acute Chronic Obstructive Pulmonary Disease (COPD) Exacerbations Despite Maximal Therapy, Oral Carbocysteine Can Be Used to Decrease the Chronic Obstructive Pulmonary Disease (COPD) Exacerbation Rate (Ungraded Consensus-Based Statement)
- Statins (see HMG-CoA Reductase Inhibitors)
- In Moderate-Severe Chronic Obstructive Pulmonary Disease (COPD) at Risk for Exacerbation, Statins are Not Recommended to Decrease the Chronic Obstructive Pulmonary Disease (COPD) Exacerbation Rate (Grade 1B Recommendation)
- Vaccination
- Risk of Future Exacerbations (Global Initiative for Chronic Obstructive Lung Disease/GOLD. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease: 2026 Report) [LINK]
- Score to Predict Short-Term Risk of COPD Exacerbation (SCOPEX)
- COPDEX-Health Search Score (CEX-HScore)
- Dyspnea, Eosinopenia, Consolidation, Acidemia, and Atrial Fibrillation (DECAF) Score
- Prognosis
- Hospitalization for COPD Exacerbation is Associated with a Poor Prognosis and Increased Mortality Rate
Acute Respiratory Failure (see Respiratory Failure)
- Epidemiology
- COPD exacerbation is the most common cause of acute ventilatory failure in US adults
- Approximately 33% of COPD exacerbations admitted to an acute care hospital develop acute ventilatory failure: risk is highest in those with FEV1 <50% pred
- Clinical
- Acute Hypoxemic Respiratory Failure
- Acute Hypercapnic/Ventilatory Failure
- Treatment
- xxxx
