Treatment-General
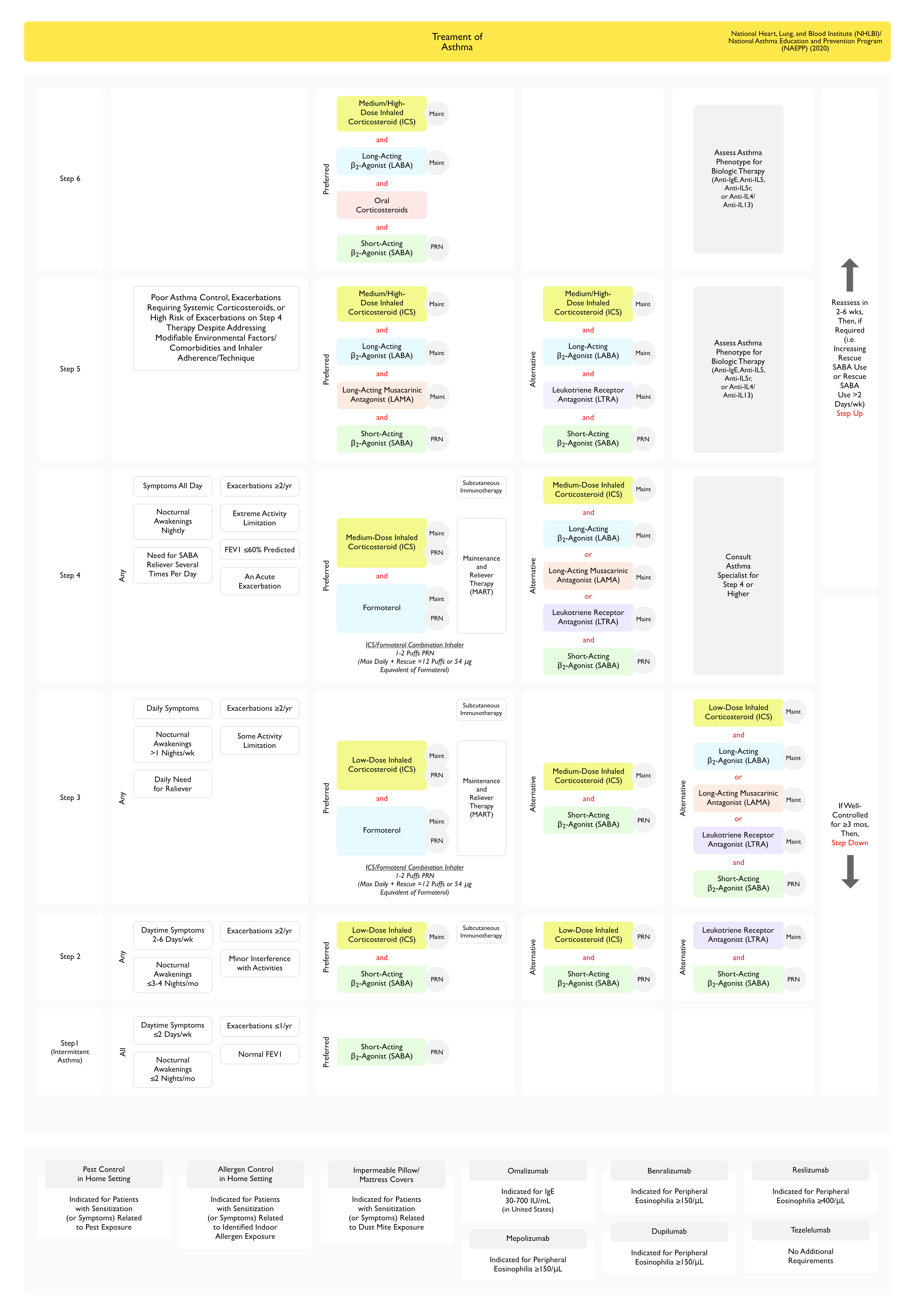
Address Underlying Factors Contributing to Asthma Pathogenesis
- Allergen Avoidance: decreases asthma severity
- Decrease Home Allergens: with ventilation, removal of pets, etc
- Lifelong Occupational Exposure Avoidance: in cases of occupational asthma
- Assure Medication Compliance: ascertain compliance with regimen
- Compliance is poorly judged by health care providers
- Compliance is related to simpler dosing regimens, but is not related to educational level, drug route (PO vs. MDI), or disease chronicity
- Avoid Dietary Sulfites
- Prevention of Infection
- Avoidance of Crowds
- Influenza Vaccination (see Influenza Virus)
- Pneumococcal Vaccination (see Streptococcus Pneumoniae)
- Tobacco Cessation (see Tobacco)
- Treat Atopy and Allergic Rhinitis (see Allergic Rhinitis): treatment of concomitant allergic rhinitis with nasal steroids improves both airway reactivity AND asthma symptoms
- Treat Chronic Rhinosinusitis (see Chronic Rhinosinusitis)
- Treat Gastroesophageal Reflux Disease (GERD) (see Gastroesophageal Reflux Disease)
- When GERD is diagnosed by pH monitoring, randomized studies suggest that omeprazole improves nocturnal (but not daytime) control (Chest 1999: 116: 5: 1257-1264)
- GERD is often clinically silent
- Treat Nasal Polyps (see Nasal Polyps): inhaled steroids are mainstay of therapy (leukotriene antagonists are not effective)
- Anosmia and/or Obstruction: systemic steroids (for 3 wks) are indicated (as inhaled steroids alone are ineffective) -> if steroid burst is ineffective, surgery
- Post-Polypectomy: post-op ASA desensitization delays polyp recurrence for up to 6 yrs
- Treat Obesity (see Obesity): if present
- Treat Obstructive Sleep Apnea (OSA) (see Obstructive Sleep Apnea): if present
- Treat Paradoxical Vocal Fold Motion (Vocal Cord Dysfunction) (see Paradoxical Vocal Fold Motion): if present
- Treat Psychological Factors (Anxiety/Depression) (see Depression)
Asthma Biomarkers, Associated Phenotypes, and Predictors of Response to Asthma Therapies
- Elevated Exhaled Nitric Oxide (FeNO): >50 ppb in adults, >35 ppb in children
- Asthma Phenotype: Th2 High
- Predicts Clinical Response to Inhaled Corticosteroids (see Corticosteroids)
- Elevated Total IgE: >30 IU
- Asthma Phenotype: Allergic
- Predicts Clinical Response to Anti-IgE Therapy
- Omalizumab (see Omalizumab)
- Lack of Peripheral/Sputum Eosinophilia and Low FeNO
- Asthma Phenotype: Th2 Low
- Predicts Clinical Response to Tiotropium (Spiriva) (see Tiotropium)
- Predicts Clinical Response to Macrolides (see Macrolides)
- Predicts Poor Clinical Response to Inhaled Corticosteroids (see Corticosteroids)
- Periostin
- Asthma Phenotype: Th2 High
- Predicts Clinical Response to Anti-IL-4 Receptor Therapy
- Dupilumab (see Dupilumab): fully human monoclonal antibody that binds to the alpha subunit of the IL-4 receptor -> inhibits downstream signaling of both IL-4 and IL-13
- Predicts Clinical Response to Anti-IL-13 Therapy
- Lebrikizumab (TNX-650) (see Lebrikizumab)
- Peripheral Eosinophilia (see Peripheral Eosinophilia): 0.3 x 10 to the 9th/L or 300/µL
- Asthma Phenotype: Th2 High
- Predicts Clinical Response to Anti-IL-5 Therapy
- Mepolizumab (Nucala) (see Mepolizumab)
- Reslizumab (Cinqair) (see Reslizumab)
- Positive Allergy Skin Tests and Elevated Specific IgE
- Asthma Phenotype: Allergic Asthma with Atopy
- Predicts Clinical Response to Allergen Immunotherapy (see Allergen Immunotherapy)
- Predicts Clinical Response to Anti-IgE Therapy
- Omalizumab (see Omalizumab)
- Sputum Eosinophilia: >3%
- Asthma Phenotype: Th2 High
- Predicts Clinical Response to Inhaled Corticosteroids (see Corticosteroids)
GINA Guidelines
- xxx
- Intermittent Asthma and Risk of Severe Exacerbation in Children. Chest. 2025 Aug 27:S0012-3692(25)05124-4. doi: 10.1016/j.chest.2025.07.4089 [MEDLINE]
- Background: Because of risk of severe asthma exacerbations, current GINA recommendations advise against use of short acting beta agonists (SABA) alone as first step in mild asthma. It is unclear if everyone with mild asthma carries equal risk for severe asthma exacerbations
- Research question: Is there a subgroup of patients with mild asthma with very low risk of severe asthma exacerbations?
- Study design and methods: Our study cohort utilized administrative claims data for patients ages 2-18 years with intermittent asthma enrolled in Ohio Medicaid Managed Care Plans for three consecutive years. For the first two years, we identified a low-risk group. In the third year, we compared risk of severe asthma exacerbations among our low-risk group and rest of the cohort
- Results: 13,208 patients met inclusion criteria. In the third year, among 3,935 low-risk patients, rates of asthma hospitalization, ED and UC visits for those with 0-2 SABA canister dispensing/year were 3 (0.08%), 37 (0.97%) and 21 (0.55%), respectively, with relative risk of hospitalization 0.17 (95% CI: 0.06, 0.52) and relative risk of severe asthma exacerbation 0.18 (95% CI: 0.13, 0.27) compared to high-risk patients. In our low-risk cohort, the number of patients needed to treat to prevent one hospitalization was 5,535. The cost to prevent one hospitalization using a single inhaler of ICS/year was $779,716
- Interpretation: Among mild asthma patients, there is a subgroup of low-risk patients with lower risk of hospitalization and severe asthma exacerbation in which current GINA recommendations for first step may neither be needed nor cost effective
Allergen Immunotherapy (see Allergen Immunotherapy)
- Indications
- Asthma Associated with Atopy
- Techniques
- Oral
- Subcutaneous
- Clinical Efficacy
- Systematic Review (88 Trials) of Injection Allergen Immunotherapy in Asthma (Cochrane Database Syst Rev, 2010) [MEDLINE]
- Trials Used Multiple Allergens: House Dust Mites, Pollen, Animal Dander, Cladosporium Mold, and Latex
- Allergen Immunotherapy Decreased Asthma Symptoms, Medication Use, and Airway Reactivity
- Allergen Immunotherapy Had No Consistent Effect on Lung Function
- Local or Systemic Adverse Effects (Anaphylaxis) Were Observed in Trials
- Systematic Review (88 Trials) of Injection Allergen Immunotherapy in Asthma (Cochrane Database Syst Rev, 2010) [MEDLINE]
Antihistamines (see H1-Histamine Receptor Antagonists)
Indications
- Asthma Associated with Allergic Rhinitis (see Allergic Rhinitis)
- Asthma Associated with Grass Pollen
Agents
- Cetirizine (Zyrtec) (see Cetirizine)
- Desloratadine (Clarinex) (see Desloratadine)
- Fexofenadine (Allegra, Axodin) (see Fexofenadine)
- Levocetirizine (Xyzal) (see Levocetirizine)
- Loratidine (Claritin) (see Loratidine)
Clinical Efficacy
- xxx
Asthma Action Plans
- xxx
- A Digital Asthma Self-Management Program for Adults: A Randomized Clinical Trial. JAMA Netw Open. 2025 Jul 1;8(7):e2521438. doi: 10.1001/jamanetworkopen.2025.21438 [MEDLINE]
- Importance: Digital health technologies may improve asthma self-management, but evidence is limited in this area
- Objective: To investigate the effect of a digital asthma self-management (DASM) program on asthma symptoms in adults
- Design, setting, and participants: Patient-reported outcome results were reported from a randomized, pragmatic, parallel-arm, open-label, decentralized clinical trial. Adults with asthma were recruited via email, enrolled from October 29, 2020, through November 4, 2021, and were randomized to DASM or usual care (control). Participants completed study activities outside a clinical setting. Data were analyzed between October 13, 2023, and November 29, 2024
- Intervention: The app-based DASM program provided tailored notifications, symptom logging, wearable device integration, and other tools
- Main outcomes and measures: Change in the Asthma Control Test (ACT) was a primary outcome. The ACT is a validated measure of asthma control. Secondary outcomes included engagement and self-reported medication adherence
- Results: Nine hundred and one participants were enrolled, with data available for 899 (639 [71.1%] female; mean [SD] age, 36.6 [10.5] years). For subgroup analyses, 195 participants (21.7%) were African American; 125 (13.9%), Hispanic or Latino; 680 (75.6%), commercially insured; and 219 (24.4%), Medicaid insured. Prespecified analyses of participants with uncontrolled asthma at baseline (n = 550) showed improvements after 12 months by 4.6 (95% CI, 4.1-5.2) ACT points among DASM participants (P < .001) and 1.8 (95% CI, 1.3-2.4) ACT points among controls (P < .001) (adjusted difference, 2.8 [95% CI, 2.0-3.6] points; P < .001). Race moderated this effect. At 12 months, the difference between arms in ACT change favored DASM over control by 1.0 (95% CI, -0.7 to 2.7) points (P = .26) for African American participants and 3.3 (95% CI, 2.4-4.2) points (P < .001) for participants not endorsing African American race (adjusted difference, -2.3 [95% CI, -4.2 to -0.4] points; P = .02 for interaction). Moderation was not observed by insurance (Medicaid vs commercial; adjusted difference, 1.0 [95% CI, -0.8 to 2.8] points; P = .18 for interaction) or ethnicity (Hispanic or Latino vs non-Hispanic; adjusted difference, 1.0 [95% CI, -1.3 to 3.3] points; P = .70 for interaction)
- Conclusions and relevance: In this randomized clinical trial of DASM, improved asthma control was observed relative to usual care. Program adaptations may be appropriate to confer benefit throughout diverse populations.
Cromones (Cromoglycates)
- Indications
- xxx
- Agents
- Cromolyn (see Cromolyn)
- Nedocromil (see Nedocromil)
- Pharmacology: xxx
- Adverse Effects: no long-term adverse effects
- Nedocromil: bad taste
- Clinical Efficacy
- xxx
- Protect sensory nerve endings/minimal inhibition of mast cell degranulation/decrease B-cell IgE production/ inhibit early, late allergic responses (late response may play role in disease severity)
- Nedocromil also inhibits eosinophils
- Protect against: nebulized water, exercise, allergens, cold air hyperventilation, SO2, bradykinin
- Clinical Uses: require regular 3-4x per day use for weeks before effect is seen
- Exercise-induced asthma
- Mild, episodic, or seasonal asthma (especially birch pollen asthma)
- Little effect on severity of persistent asthma (despite effect on late allergic response)
- May provide some protection against ASA-induced asthma
- Nedocromil more effective than Cromolyn in adults (may produce some reduction in steroid dose)
- No evidence that cromolyn provides additional benefit in patients already on high-dose inhaled steroids
Short-Acting β2-Adrenergic Receptor Agonists (SABA) (see β2-Adrenergic Receptor Agonists)
Agents
- Albuterol (Salbutamol, Ventolin) (see Albuterol)
- Levalbuterol (Xopenex) (see Levalbuterol)
- Pirbuterol (Maxair) (see Pirbuterol)
Pharmacology
- ß2 adrenergic receptor agonism -> inhibit mast cell mediator release, relax airway smooth muscle (dose-related protection against osmotics, allergens, exercise, histamine, methacholine, SO2), increase mucus clearance, protect venular endothelial cells/ long-term use may impair healing after acute allergic reaction (possibly due to less mast cell heparin release)
- PO dosing of short-acting ß2-agonists: more systemic SE, less protection/may be useful in some cases though for nocturnal symptoms
- Regular use of short-acting ß2-agonists: may worsen asthma (therefore, should use these intermittently, as rescue medications)
- Normals but not asthmatics become tolerant to bronchodilation with regular use
- Regular use of salbutamol/fenoterol shown to decrease lung function, worsen hyperreactivity, and worsen disease control
Clinical Efficacy-General
- Systematic Review and Meta-Analysis of XXXXX (Allergy, 2025) [MEDLINE]
- XXXX
- Adverse Outcomes Associated With Short-Acting Beta-Agonist Overuse in Asthma: A Systematic Review and Meta-Analysis. Allergy. 2025 Jun 10. doi: 10.1111/all.16538 [MEDLINE]
- Introduction: The 2019 Global Initiative for Asthma (GINA) report no longer recommended short-acting beta-agonists (SABA) monotherapy due to associated complications and a lack of anti-inflammatory properties. This systematic review and meta-analysis aimed to evaluate adverse outcomes associated with SABA overuse in patients with asthma
- Methods: PubMed, Cochrane Library, EMBASE, and Web of Science databases were searched for studies on SABA overuse (≥ 3 SABA canisters/year) in patients with asthma, from 1981 to November 2023. Randomized controlled trials (RCTs), cohort studies, and cross-sectional studies were included. Pooled risk ratios (RRs) were calculated for dichotomous measures of all-cause mortality and acute exacerbations using random-effects models and Mantel-Haenszel weighting. Subgroup analyses were conducted based on study design
- Results: Out of 626 records, 27 studies (2 RCTs, 1 prospective cohort study, 12 retrospective cohort studies, and 12 cross-sectional studies) were included. SABA overuse (≥ 3 SABA canisters/year) was associated with significantly higher mortality (2743 of 130,629 in the overuse group versus 3534 of 300,451 in controls; RR = 2.04, 95% confidence interval, CI = 1.37-3.04; p < 0.001) and a significantly higher rate of acute exacerbations (60,320 of 165,271 in the overuse group versus 84,439 of 376,845 in controls; RR = 1.93, 95% CI = 1.24-3.03; p < 0.001). An increased risk of acute exacerbations was observed in retrospective cohort studies (RR = 1.88, 95% CI = 1.43-2.47; p < 0.001) and cross-sectional studies (RR = 2.23, 95% CI = 1.04-4.77; p < 0.001)
- Conclusions: SABA overuse was associated with increased rates of mortality and acute exacerbations in patients with asthma, supporting guidelines that advise against SABA monotherapy in asthma management.
Clinical Efficacy-As Needed β2-Agonists with/without As Needed Inhaled Corticosteroids
- BATURA Trial (NEJM, 2025) [MEDLINE]
- XXXX
- As-Needed Albuterol-Budesonide in Mild Asthma. N Engl J Med. 2025 Jul 10;393(2):113-124. doi: 10.1056/NEJMoa2504544 [MEDLINE]
- Background: As-needed use of albuterol-budesonide has been shown to result in a significantly lower risk of severe asthma exacerbation than as-needed use of albuterol alone among patients with moderate-to-severe asthma. Data on albuterol-budesonide in mild asthma are needed
- Methods: We conducted a fully virtual, decentralized, phase 3b, multicenter, double-blind, event-driven trial involving persons 12 years of age or older with disease that was uncontrolled despite treatment for mild asthma with a short-acting β2-agonist (SABA) with or without a low-dose inhaled glucocorticoid or leukotriene-receptor antagonist. Participants were randomly assigned in a 1:1 ratio to a fixed-dose combination of 180 μg of albuterol and 160 μg of budesonide (with each dose consisting of two inhaler actuations of 90 μg and 80 μg, respectively) or 180 μg of albuterol (with each dose consisting of two inhaler actuations of 90 μg) on an as-needed basis for up to 52 weeks. The primary end point was the first severe asthma exacerbation, assessed in a time-to-event analysis, in the on-treatment efficacy population, and the key secondary end point was the first severe exacerbation in the intention-to-treat population. Secondary end points included the annualized rate of severe asthma exacerbations and exposure to systemic glucocorticoids
- Results: A total of 2516 participants underwent randomization; 1797 (71.4%) completed the trial. Of 2421 participants in the full analysis population (1209 assigned to the albuterol-budesonide group and 1212 to the albuterol group), 97.2% were 18 years of age or older; 74.4% used a SABA alone at baseline. The trial was stopped for efficacy at a prespecified interim analysis. A severe exacerbation occurred in 5.1% of the participants in the albuterol-budesonide group and in 9.1% of those in the albuterol group in the on-treatment efficacy population (hazard ratio, 0.53; 95% confidence interval [CI], 0.39 to 0.73) and in 5.3% and 9.4%, respectively, in the intention-to-treat population (hazard ratio, 0.54; 95% CI, 0.40 to 0.73) (P<0.001 for both comparisons). The annualized rate of severe asthma exacerbations was lower with albuterol-budesonide than with albuterol (0.15 vs. 0.32; rate ratio, 0.47; 95% CI, 0.34 to 0.64), as was the mean annualized total dose of systemic glucocorticoids (23.2 vs. 61.9 mg per year). Adverse events were similar in the two treatment groups
- Conclusions
- As-needed use of albuterol-budesonide resulted in a lower risk of a severe asthma exacerbation than as-needed use of albuterol alone among participants with disease that was uncontrolled despite treatment for mild asthma
Long-Acting β2-Adrenergic Receptor Agonists (LABA) (see β2-Adrenergic Receptor Agonists)
Long-Acting ß2-Adrenergic Agonists (LABA) Agents
- Arformoterol (Brovana, Erdotin) (see Arformoterol)
- Bambuterol (Bambec, Oxeol)
- Clenbuterol (Spiropent, Ventipulmin, Dilaterol, Spiropent) (see Clenbuterol)
- Formoterol (Foradil, Oxeze, Oxis, Atock, Atimos, Perforomist) (see Formoterol)
- Formoterol + Budesonide (Symbicort) (see Formoterol + Budesonide)
- Olodaterol (Striverdi Respimat) (see Olodaterol)
- Olodaterol + Tiotropium (Stiolto Respimat) (see Olodaterol-Tiotropium)
- Salmeterol (Serevent) (see Salmeterol)
- Salmeterol + Fluticasone (Advair) (see Salmeterol + Fluticasone)
Ultra Long-Acting ß2-Adrenergic Agonists (Ultra LABA) Agents
- Indacaterol (Arcapta) (see Indacaterol)
- Olodaterol (see Olodaterol)
- Olodaterol + Tiotropium (Stiolto Respimat) (see Olodaterol-Tiotropium)
- Vilanterol (see Vilanterol)
- Vilanterol + Fluticasone (Breo Ellipta) (see Vilanterol + Fluticasone)
- Vilanterol + Umeclidinium (Anoro Ellipta) (see Vilanterol + Umeclidinium)
Pharmacology
- ß2-adrenergic receptor agonism: inhibit mast cell mediator release, relax airway smooth muscle (dose-related protection against osmotics, allergens, exercise, histamine, methacholine, SO2), increase mucus clearance, protect venular endothelial cells/ long-term use may impair healing after acute allergic reaction (possibly due to less mast cell heparin release)
- No evidence that regular use of long-acting ß2-agonists decreases responsiveness to short-acting ß2-agonists
Clinical Efficacy
- ß2-Adrenergic Receptor Gene (ADRB2) Variants as Predictors of Risk with Use of LABA’s in Multiethnic Asthma Population (Lancet Resp Med, 2014) [MEDLINE]
- The rare ADRB2 alleles, Ile164 and -376ins, are associated with adverse events during LABA therapy
- AUSTRI Study of Serious Events with Fluticasone + Salmeterol vs Fluticasone Alone (NEJM, 2016) [MEDLINE]
- No Difference in Serious Asthma-Related Events Between Fluticasone + Salmeterol vs Fluticasone Alone
- Fluticasone + Salmeterol Group Had Fewer Severe Asthma Exacerbations than the Fluticasone Alone Group
- VESTRI Trial Examining the Safety of Adding Salmeterol to Fluticasone Propionate in Children with Asthma (NEJM, 2016) [MEDLINE]
- In Children with Asthma, There was No Difference in Risk of Serious Asthma-Related Events Between Salmeterol + Fluticasone vs Fluticasone Alone
- Trial Examining Serious Asthma Events with Budesonide + Formoterol vs Budesonide Alone (NEJM, 2016) [MEDLINE]
- In Moderate-Severe Asthma in Adolescents/Adults, Budesonide + Formoterol was Associated with Lower Risk of Asthma Exacerbations than Budesonide Alone and a Similar Risk of Serious Asthma-Related Events
Clinical Efficacy-Stepdown Therapy
- Randomized Trial of Stepdown Protocols in Asthma (J Allergy Clin Immunol Pract, 2018) [MEDLINE]: n = 459 randomized to either medium-dose inhaled corticosteroid/LABA, reduced-dose inhaled corticosteroid/LABA, or inhaled corticosteroid alone (LABA step-off)
- Stepdown Regimens were Similar in Terms of Treatment Failure
- However, Stopping LABA Resulted in a Decline in Lung Function and More Hospitalizations
Short-Acting Antimuscarinic/Anticholinergic (SAMA) Agents (see Muscarinic Antagonists)
- Agents
- Ipratropium (Atrovent) (see Ipratropium Bromide)
- Pharmacology: short-acting muscarinic antagonist
- Clinical Efficacy
- xxxx
Long-Acting Anti-Muscarinic/Anti-Cholinergic (LAMA) Agents (see Muscarinic Antagonists)
- Agents
- Aclidinium (Tudorza Pressair) (see Aclidinium)
- Tiotropium (Spiriva) (see Tiotropium)
- Pharmacology: anti-muscarinic
- Clinical Efficacy
- Tiotropium Add-On Therapy to Corticosteroids and LABA in Asthma (NEJM, 2012) [MEDLINE]
- Tiotropium Increased Time to First Severe Exacerbation (282 Days, As Compared to 226 Days) and Resulted in a 21% Decreased Risk of Severe Exacerbations (Hazard Ratio: 0.79)
- Tiotropium Provided Modest Sustained Bronchodilation (Increased FEV1)
- Systematic Review and Meta-Analysis of Tiotropium in Asthma (Chest, 2015) [MEDLINE]
- Tiotropium was Non-Inferior to Salmeterol (and Superior to Placebo) in Patients with Moderate-Severe Asthma Who were Not Adequately Controlled on Inhaled Corticosteroids or Inhaled Corticosteroids/Salmeterol
- Major Benefits Involved an Increase in Lung Function and, with Severe Asthma, Decreased Exacerbations
- Systematic Review of LAMA in Asthma (Cochrane Database Syst Rev, 2016) [MEDLINE]
- Tiotropium Add-On Therapy May Have Additional Benefits Over Inhaled Corticosteroids/LABA Alone in Decreasing the Need for Rescue Oral Corticosteroids in Severe Asthma: no evidence is available for other LAMA’s
- Possible Benefits of Tiotropium on Quality of Life Were Negligible and Evidence for the Effect on Serious Adverse Events was Inconsistent
- Benefits of Tiotropium Add-On Therapy on the Frequency of Hospital Admissions are Unknown
- Tiotropium Add-On Therapy to Corticosteroids and LABA in Asthma (NEJM, 2012) [MEDLINE]
Leukotriene Modifier Agents
- Indications
- Excercise-Induced Asthma: however, these are less effective than inhaled corticosteroids
- Agents
- Montelukast (Singulair) (see Montelukast)
- Zafirlukast (Accolate) (see Zafirlukast)
- Zileuton (Zyflo) (see Zileuton)
- Pharmacology: xxx
- Adverse Effects: generally well-tolerated
- Rare Cases of Churg-Strauss Syndrome Have Been Reported with Montelukast and Corticosteroid Withdrawal (see Churg-Strauss Syndrome): unclear association
- Clinical Efficacy
- xxx
- Improve airway function/protect against exercise (block 60-80% of bronchoconstriction), hyperventilation, (block 60-80% of bronchoconstriction), allergen (block 80-90% of bronchoconstriction), ASA (completely block bronchoconstriction), and cold-induced asthma
- More effective when given systemically (suggests major effect occurs below epithelium)
- Decreases need for rescue ß2-agonists, improves FEV1, decreases symptoms, decreases asthma exacerbations requiring PO steroids, and decreases inhaled steroid requirements
- Leukotriene antagonists have no effect on nasal polyps
- Particularly useful: exercise-associated asthma and ASA-sensitve asthma
Theophylline (Theo-Dur) (see Theophylline)
Epidemiology
- Theophylline is the Most Widely-Used Asthma Drug Worldwide
Pharmacology
- Theophylline is a Methylxanthine
- Anti-Inflammatory Effects
- Decreased Mast Cell Mediator Release
- Diuretic
- Immunomodulatory Effects
- Increased Diaphragmatic Contractility
- Weak Bronchodilator
Administration
- Need to Monitor Theophylline Levels: target steady-state level: 5-15 ug/mL
- Serum Theophylline Levels May Be Altered by Various Factors: fever, diet/food, age, smoking, other medications
Adverse Effects
- General Comments: adverse effects are generally dose-related
- Arrhythmias
- Central Nervous System Stimulation
- Headache (see Headache)
- Hematemesis (see Gastrointestinal Hemorrhage)
- Hyperglycemia (see Hyperglycemia)
- Hypokalemia (see Hypokalemia)
- Nausea/Vomiting (see Nausea and Vomiting)
- Seizures (see Seizures)
- Sinus Tachycardia (see Sinus Tachycardia)
Potential Clinical Scenarios in Asthma Where Theophylline May Have Utility
- Acute Asthma Exacerbation in the Intensive Care Unit: data for clinical benefit in this situation are lacking
- Add-On Therapy for Disease Uncontrolled on Inhaled Corticosteroids or When LABA is Not Beneficial or Desired
- Theophylline is Probably More Efficacious Than Cromolyn/Nedocromil When Added to Inhaled Corticosteroids
- Primary Maintenance Therapy Where Compliance to an Oral Agent is More Likely, But Montelukast is Ineffective
- Primary Maintenance Therapy Where Inhaled Corticosteroid Use is Difficult (Small Children, etc), But Montelukast is Ineffective
Clinical Efficacy
- Theophylline was Less Efficacious Than Long-Acting Beta Agonists (LABA) in Asthma (Respir Med, 1998) [MEDLINE]
- Theophylline Withdrawal in Severe Asthma was Associated with an Increased Rate of Asthma Exacerbations (Am J Respir Crit Care Med, 1995) [MEDLINE]
Inhaled Corticosteroids (see Corticosteroids)
- xxxx
- Decrease severity of persistent asthma/increase dosing with more severe disease (quicker response with higher doses: symptoms respond within days, airway reactivity and PEFR respond within weeks)/use high dose x3-6 months to control, then decrease to level that prevents exacerbations (improvement continues over months)
- Target: <15% PEFR variability, decreased symptoms, etc.
- Timing of Inhaled Steroid Introduction: the FEV1 is less likely to normalize the later inhaled steroids are initiated
- Effect on Asthma Mortality: in study of 5-44 y/o patients, inhaled steroids (>6 canisters during 12-month period) result in 50% decrease in asthma mortality (effect is dose-dependent)
- SE: thrush/dysphonia (due to vocal cord myopathy)/systemic effects (potential for systemic absorption increases with dose)
- No cases of adrenal insufficiency occur at <2000 µg/day
- Steroid-resistance (failure after >6 months of inhaled steroids/possibly due to Ab against lipocortin-1, decreased monocyte receptor response): actual resistance is rare (must rule out poor drug delivery, GERD, OSA, non-compliance, etc.)
Agents
- Beclomethasone (QVAR) (see Beclomethasone)
- Budesonide (Pulmicort, Rhinocort) (see Budesonide)
- Formoterol + Budesonide (Symbicort) (see Formoterol + Budesonide)
- Ciclesonide (Alvesco) (see Ciclesonide)
- Flunisolide (xxxxxx) (see Flunisolide)
- Fluticasone (Flovent) (see Fluticasone)
- Salmeterol + Fluticasone (Advair) (see Salmeterol + Fluticasone)
- Vilanterol + Fluticasone (Breo Ellipta) (see Vilanterol + Fluticasone)
- Mometasone (Asmanex, Nasonex) (see Mometasone)
- Triamcinolone (xxxxxx) (see Triamcinolone)
Clinical Efficacy
- Adding LABA vs to Inhaled Corticosteroids
- FACET Trial (NEJM, 1997) [MEDLINE]
- Adding LABA Instead of Increasing Inhaled Corticosteroids Alone Decreased Asthma Exacerbations and Improved PEFR in Moderate Asthma
- AUSTRI Study of Serious Events with Fluticasone + Salmeterol vs Fluticasone Alone (NEJM, 2016) [MEDLINE]
- No Difference in Serious Asthma-Related Events Between Fluticasone + Salmeterol vs Fluticasone Alone
- Fluticasone + Salmeterol Group Had Fewer Severe Asthma Exacerbations than the Fluticasone Alone Group
- VESTRI Trial Examining the Safety of Adding Salmeterol to Fluticasone Propionate in Children with Asthma (NEJM, 2016) [MEDLINE]
- In Children with Asthma, There was No Difference in Risk of Serious Asthma-Related Events Between Salmeterol + Fluticasone vs Fluticasone Alone
- Trial Examining Serious Asthma Events with Budesonide + Formoterol vs Budesonide Alone (NEJM, 2016) [MEDLINE]
- In Moderate-Severe Asthma in Adolescents/Adults, Budesonide + Formoterol was Associated with Lower Risk of Asthma Exacerbations than Budesonide Alone and a Similar Risk of Serious Asthma-Related Events
- FACET Trial (NEJM, 1997) [MEDLINE]
- Variability in Individual Responsiveness to Inhaled Corticosteroids
- Variability in Individual Responsiveness to Inhaled Corticosteroids Can Be Predicted by Single Nucleotide Polymorphisms (SNP) in the T Gene (Am J Respir Crit Care Med, 2012) [MEDLINE]
- Approximate 2-3x Differences in FEV1 Response Were Observed for Subjects Homozygous for Wild-Type vs Mutant Alleles for Each T Gene SNP
- Variability in Individual Responsiveness to Inhaled Corticosteroids Can Be Predicted by Single Nucleotide Polymorphisms (SNP) in the T Gene (Am J Respir Crit Care Med, 2012) [MEDLINE]
- Use of Inhaled Corticosteroids in Asthma and Risk of Pneumonia
- Retrospective Analysis of Risk of Pneumonia in Asthma Patients on Budesonide (Am J Respir Crit Care Med, 2011) [MEDLINE]
- Budesonide Use in Asthma Did Not Increase the Risk of Pneumonia
- Case-Control Study from Health Improvement Network (Chest, 2013) [MEDLINE]
- Inhaled Corticosteroids Dose-Dependently Increased the Risk of Pneumonia
- Retrospective Analysis of Risk of Pneumonia in Asthma Patients on Budesonide (Am J Respir Crit Care Med, 2011) [MEDLINE]
- Use of Inhaled Corticosteroids and the Risk of Developing Parapneumonic Effusion in Association with Pneumonia
- Spanish Study of the Effect of Prior Inhaled Corticosteroids (in Both Asthma and COPD) on the Risk of Developing Parapneumonic Effusion in Association with Pneumonia (Am J Respir Crit Care Med, 2013) [MEDLINE]
- Prior Use of Inhaled Corticosteroids Decreased the Risk of Parapneumonic Effusion in Association with Pneumonia
- Prior Use of Inhaled Corticosteroids was Associated with Higher Pleural pH, Higher Pleural Glucose, Lower Pleural Protein, and Lower Pleural LDH
- Spanish Study of the Effect of Prior Inhaled Corticosteroids (in Both Asthma and COPD) on the Risk of Developing Parapneumonic Effusion in Association with Pneumonia (Am J Respir Crit Care Med, 2013) [MEDLINE]
- Inhaled Corticosteroid Use in Children and Effects on Growth
Clinical Efficacy-Stepdown Therapy
- Randomized Trial of Stepdown Protocols in Asthma (J Allergy Clin Immunol Pract, 2018) [MEDLINE]: n = 459 randomized to either medium-dose inhaled corticosteroid/LABA, reduced-dose inhaled corticosteroid/LABA, or inhaled corticosteroid alone (LABA step-off)
- Stepdown Regimens were Similar in Terms of Treatment Failure
- However, Stopping LABA Resulted in a Decline a Lung Function and More Hospitalizations
Clinical Efficacy-As Needed β2-Agonists with/without As Needed Inhaled Corticosteroids
- BATURA Trial (NEJM, 2025) [MEDLINE]
- XXXX
- As-Needed Albuterol-Budesonide in Mild Asthma. N Engl J Med. 2025 Jul 10;393(2):113-124. doi: 10.1056/NEJMoa2504544 [MEDLINE]
- Background: As-needed use of albuterol-budesonide has been shown to result in a significantly lower risk of severe asthma exacerbation than as-needed use of albuterol alone among patients with moderate-to-severe asthma. Data on albuterol-budesonide in mild asthma are needed
- Methods: We conducted a fully virtual, decentralized, phase 3b, multicenter, double-blind, event-driven trial involving persons 12 years of age or older with disease that was uncontrolled despite treatment for mild asthma with a short-acting β2-agonist (SABA) with or without a low-dose inhaled glucocorticoid or leukotriene-receptor antagonist. Participants were randomly assigned in a 1:1 ratio to a fixed-dose combination of 180 μg of albuterol and 160 μg of budesonide (with each dose consisting of two inhaler actuations of 90 μg and 80 μg, respectively) or 180 μg of albuterol (with each dose consisting of two inhaler actuations of 90 μg) on an as-needed basis for up to 52 weeks. The primary end point was the first severe asthma exacerbation, assessed in a time-to-event analysis, in the on-treatment efficacy population, and the key secondary end point was the first severe exacerbation in the intention-to-treat population. Secondary end points included the annualized rate of severe asthma exacerbations and exposure to systemic glucocorticoids
- Results: A total of 2516 participants underwent randomization; 1797 (71.4%) completed the trial. Of 2421 participants in the full analysis population (1209 assigned to the albuterol-budesonide group and 1212 to the albuterol group), 97.2% were 18 years of age or older; 74.4% used a SABA alone at baseline. The trial was stopped for efficacy at a prespecified interim analysis. A severe exacerbation occurred in 5.1% of the participants in the albuterol-budesonide group and in 9.1% of those in the albuterol group in the on-treatment efficacy population (hazard ratio, 0.53; 95% confidence interval [CI], 0.39 to 0.73) and in 5.3% and 9.4%, respectively, in the intention-to-treat population (hazard ratio, 0.54; 95% CI, 0.40 to 0.73) (P<0.001 for both comparisons). The annualized rate of severe asthma exacerbations was lower with albuterol-budesonide than with albuterol (0.15 vs. 0.32; rate ratio, 0.47; 95% CI, 0.34 to 0.64), as was the mean annualized total dose of systemic glucocorticoids (23.2 vs. 61.9 mg per year). Adverse events were similar in the two treatment groups
- Conclusions
- As-needed use of albuterol-budesonide resulted in a lower risk of a severe asthma exacerbation than as-needed use of albuterol alone among participants with disease that was uncontrolled despite treatment for mild asthma
Systemic Corticosteroids (see Corticosteroids)
Indications
- Acute Asthma Exacerbation/Status Asthmaticus
Administration
- General Comments
- Intravenous Onset of Action is Probably Faster than Oral Onset
- Probably Do Not Need to Stop Inhaled Corticosteroids During an Acute Asthma Exacerbation
- PO (Usually Prednisone)
- IV (Usually Methylprednisolone as Solumedrol)
- IM (Usually Methylprednisolone as Medrol):
Pharmacology
- Anti-Inflammatory
Adverse Effects
- Cataracts
- Hyperglycemia (see Hyperglycemia)
- Immunosuppression
- Myopathy (see Myopathy)
- Other
Drug Interactions
- Antacids: decrease gastrointestinal corticosteroid absorption
- Rifampin/Phenobarbital/Phenytoin/Ephedrine: via effects on hepatic P-450 metabolism, these drugs increase corticosteroid clearance
Clinical Efficacy
- Post-Corticosteroid Course PEFR Provides “Best” PEFR for Future Reference
- Prevention of Cardiovascular and Other Systemic Adverse Outcomes in Patients with Asthma Treated with Biologics. Am J Respir Crit Care Med. 2025 Jul;211(7):1165-1174. doi: 10.1164/rccm.202501-0246OC [MEDLINE]
- Rationale: Although clinical trials have documented the oral corticosteroid (OCS)-sparing effect of biologics in patients with severe asthma, little is known about whether this translates to a reduction of new-onset OCS-related adverse outcomes
- Objective: To compare the risk of developing new-onset OCS-related adverse outcomes between biologic initiators and noninitiators
- Methods: This was a longitudinal cohort study using pooled data from the International Severe Asthma Registry (ISAR; 16 countries) and the Optimum Patient Care Research database (OPCRD; United Kingdom). For biologic initiators, the index date was the date of biologic initiation. For noninitiators, it was the date of enrollment (for ISAR) or a random medical appointment date (for OPCRD). Inverse probability of treatment weighting was used to improve comparability between groups, and weighted Cox proportional hazard models were used to estimate the hazard ratios (HRs) of developing OCS-related adverse outcomes for up to 5 years from the index date
- Measurements and Main Results: A total of 42,908 patients were included. Overall, 27.3% and 4.7% of biologic initiators and noninitiators were long-term OCS users (daily intake ⩾90 consecutive days in year before the index date), with a mean prednisolone-equivalent daily dose of 10.2 mg and 6.2 mg, respectively. Compared with noninitiators, biologic initiators had decreased rate of developing any OCS-related adverse outcome (HR [95% confidence interval (CI)]: 0.82 [0.72-0.93]; P = 0.002), primarily driven by reduced rate of developing diabetes (0.62 [0.45-0.87]; P = 0.006), major cardiovascular events (0.65 [0.44-0.97]; P = 0.034), and anxiety and/or depression (0.68 [0.55-0.85]; P = 0.001). There were no significant differences in the rates of new-onset cataract (HR, 0.77 [95% CI, 0.47-1.25]), sleep apnea (HR, 0.82 [95% CI, 0.78-1.41]), or other OCS-related adverse outcomes assessed (e.g., osteoporosis). The results were consistent across both datasets
- Conclusions: Our findings highlight the role for biologics in preventing new-onset OCS-related adverse outcomes in patients with severe asthma.
Omalizumab (Xolair) (see Omalizumab)
Indications
- Asthma with Positive Allergy Skin Tests and Elevated Specific IgE: indicates allergic asthma with atopy phenotype
- Asthma with Elevated IgE (>30 IU): indicates allergic asthma phenotype
Pharmacology
- Anti-Immunoglobulin E (Anti-IgE)
Administration
- xxx
Adverse Effects
- Injection Site Reaction
Clinical Efficacy
- Trial of Omalizumab for Asthma in Inner-City Children (NEJM, 2011) [MEDLINE]: omalizumab added to standard asthma therapy in inner-city children/adolescents/young adults
- Omalizumab Decreased the Number of Days with Asthma Symptoms
- Omalizumab Decreased Asthma Exacerbations: nearly eliminated seasonal peaks in exacerbations
- Omalizumab Decreased the Need for Other Medications to Control Asthma
- Cochrane Database Systematic Review Examining Omalizumab in Adults/Children with Asthma (2014) [MEDLINE]
- As Adjunct to Inhaled Corticosteroids (and During Corticosteroid Tapering), Omalizumab Decreased Asthma Exacerbations/Hospitalizations
- Omalizumab was Effective in Allowing Corticosteroid Taper/Withdrwal
- Unclear Whether There is a Threshold Level of Serum IgE Which Predicts Optimal Efficacy of Omalizumab
Anti-Interleukin-4 (IL-4) Therapy
Dupilumab (Dupixent) (see Dupilumab)
- Indications
- Asthma with Elevated Periostin Level (see Serum Periostin)
- Pharmacology: fully human monoclonal antibody that binds to the alpha subunit of the IL-4 receptor -> inhibits downstream signaling of both IL-4 and IL-13
- Clinical Efficacy
- Multi-Center Phase 2 Trial of Dupilumab in Adults with Moderate-Severe Asthma (NEJM, 2013) [MEDLINE]
- Study Indications in Phase 2 Trial in Adults with Mild-Moderate Severe Asthma
- Peripheral Eosinophilia: ≥300 cells/µL
- Sputum Eosinophilia: ≥3%
- Symptoms Not Well-Controlled on Medium-High Dose Inhaled Corticosteroids and Long-Acting Beta Agonist (LABA)
- Dupilumab Decreased Asthma Exacerbations When Corticosteroids and LABA Were Withdrawn: improved lung function and decreased Th2-associated inflammatory markers
- Study Indications in Phase 2 Trial in Adults with Mild-Moderate Severe Asthma
- Multi-Center Phase 2 Trial of Dupilumab in Adults with Moderate-Severe Asthma (NEJM, 2013) [MEDLINE]
- Dupilumab Reduces Oral Corticosteroid Use in Patients With Corticosteroid-Dependent Severe Asthma. An Analysis of the Phase 3, Open-Label Extension TRAVERSE Trial. Chest. 2022 Jul;162(1):46-55. doi: 10.1016/j.chest.2022.01.071 [MEDLINE]
- Background: Many patients with severe asthma require chronic corticosteroid treatment to maintain asthma control
- Research question: Are the reduction in oral corticosteroid (OCS) use and the clinical efficacy observed with dupilumab treatment maintained long-term in patients with severe OCS-dependent asthma?
- Study design and methods: The LIBERTY ASTHMA TRAVERSE study (ClinicalTrials.gov identifier: NCT02134028) was a multinational, multicenter, single-arm, open-label extension study in patients ≥ 12 years of age with asthma who participated in previous dupilumab studies. Treatment consisted of dupilumab 300 mg every 2 weeks for up to 96 weeks. In this analysis, we present the data from patients who initially enrolled in the LIBERTY ASTHMA VENTURE study (ClinicalTrials.gov identifier: NCT02528214), a 24-week placebo-controlled study of dupilumab in patients with OCS-dependent severe asthma, and continued in the TRAVERSE study. The subgroups analyzed were: those who received dupilumab in both (dupilumab/dupilumab group) and those who received placebo in the VENTURE study and dupilumab in the TRAVERSE study (placebo/dupilumab group). Outcomes included OCS use, exacerbation rates, and measures of lung function and asthma control
- Results: Ninety patients treated with dupilumab/dupilumab and 97 patients treated with placebo/dupilumab in the VENTURE study were enrolled and treated in the TRAVERSE study, with a mean OCS dosage of 11.0 mg/d (dupilumab) and 11.6 mg/d (placebo) at VENTURE study baseline. At TRAVERSE week 0, the mean daily OCS dosage was 3.1 mg/d and 6.4 mg/d (percentage decrease from the VENTURE study baseline, 68.8% and 41.3%) for the dupilumab/dupilumab group and placebo/dupilumab group, respectively, and decreased to 2.2 mg/d and 4.9 mg/d (78.3% and 53.4%) at week 48 and to 1.2 mg/d and 3.0 mg/d (89.3% and 74.4%) at week 96, respectively. Exacerbation rates were low during the TRAVERSE study. Further improvements from the VENTURE to TRAVERSE studies also were seen in FEV1 and 5-item Asthma Control Questionnaire scores. Safety findings were consistent with the known dupilumab safety profile
- Interpretation: In the open-label TRAVERSE study, dupilumab demonstrated the ability to sustain the OCS dosage reduction from the parent OCS-sparing study, while maintaining a low exacerbation rate and improved lung function.
- The Role of Bronchial Biopsy in the Prediction of Response to Biologic Therapy in Severe Uncontrolled Asthma: A Prospective Study. Chest. 2025 Apr;167(4):945-955. doi: 10.1016/j.chest.2024.11.045 [MEDLINE]
- Background: Up to two-thirds of patients with severe uncontrolled asthma (SUA) who received biologic therapy do not have a complete response
- Research question: Can bronchial biopsy (BB) play a role in the identification of patients with SUA who have a better response to biologic therapy?
- Study design and methods: This prospective multicenter study included consecutive patients with SUA who were candidates for biologic therapy. They underwent bronchoscopy and BB prior to biologic therapy, and clinical response was evaluated 6 months later. BB was evaluated according to a previously validated pathological score (PS) and was compared with a score of type 2 (T2) inflammation (T2 score) that includes blood eosinophil count and fractional exhaled nitric oxide in predicting response to biologic therapy. Response was graded as super-response, good response, and partial/no response according to a composite score that includes exacerbations, oral corticosteroid steroid (OCS) use, asthma control test, and improvement in FEV1
- Results: A total of 92 patients were recruited. Of the 92 patients recruited, 78 completed the study. Among them, 63 received an anti-IL-5 or IL-5 receptor (anti-IL5/5R) (mepolizumab, reslizumab, and benralizumab) while 15 received dupilumab. The proportion of super-responders was 36.5% in the anti-IL5/5R group and 26.6% in the dupilumab group (P = .126). The PS was the only variable independently associated with response; the T2 score was not. Super-responders had a statistically significantly higher PS. Response was better predicted by the PS compared with the T2 score in those receiving OCSs and especially in those taking anti-IL5/5Rs. Reduced eosinophil levels (< 10 eosinophils/field) were associated with poor response to biologic therapy
- Interpretation: Our findings indicate that BB is more precise in the prediction of response to biologic therapy than the T2 score, especially in those requiring OCSs or receiving anti-IL5/5Rs. Tissue eosinophilia is the main driver of this predictive capacity. However, other items in the PS related to bronchial remodeling might contribute to the identification of response to biologic therapy.
Anti-Interleukin-5 (IL-5) Therapy
Mepolizumab (Nucala) (see Mepolizumab)
- Indications
- Asthma with Eosinophilic Inflammation (Peripheral Eosinophilia >300/µL)
- FDA-Approved for Maintenance Therapy in Severe Asthma in Patients Age 12 and Older
- Asthma with Eosinophilic Inflammation (Peripheral Eosinophilia >300/µL)
- Pharmacology: humanized monoclonal antibody against interleukin-5 (IL-5) -> decreases the production and survival of eosinophils
- Administration: 100 mg SQ (approximate cost: $3000)
- Adverse Effects
- Headache (see Headache): occurs in 19% of cases
- Herpes Zoster Infection (see Varicella-Zoster Virus)
- Hypersensitivity Reaction: typically occur within hours of administration
- Angioedema (see Angioedema)
- Bronchospasm (see Obstructive Lung Disease)
- Hypotension (see Hypotension)
- Urticaria (see Urticaria)
- Unknown Impact on Immune Response to Helminth Infections
- Clinical Efficacy
- DREAM Trial in Asthma with Eosinophilic Inflammation (Sputum Eosinophilia, Peripheral Eosinophilia, Increased Exhaled NO, or Decreased Control with Inhaled/Systemic Steroid Taper) (Severe Eosinophilic Asthma) (Lancet, 2012) [MEDLINE]
- Mepolizumab Decreased Asthma Exacerbations in Severe Eosinophilic Asthma
- MENSA Trial of Mepolizumab in Asthma with Eosinophilic Inflammation (Severe Eosinophilic Asthma) (Peripheral Eosinophilia) Without Control on High-Dose Inhaled Corticosteroids (NEJM, 2014) [MEDLINE]
- Mepolizumab (IV, SQ) Decreased Asthma Exacerbations
- Mepolizumab Improved FEV1
- Mepolizumab Improved 5-Item Asthma Control Questionnaire Scores (ACQ-5): marker of asthma symptom control
- Mepolizumab Improved St. George’s Respiratory Questionnaire (SGRQ) Scores
- SIRIUS Trial in Asthma with Eosinophilic Inflammation (Peripheral Eosinophilia) Treated with Systemic Corticosteroids (NEJM, 2014) [MEDLINE]
- Mepolizumab Decreased Asthma Exacerbations
- Mepolizumab had a Significant Glucocorticoid-Sparing Effect
- Mepolizumab Improved 5-Item Asthma Control Questionnaire Scores (ACQ-5): marker of asthma symptom control
- DREAM Trial in Asthma with Eosinophilic Inflammation (Sputum Eosinophilia, Peripheral Eosinophilia, Increased Exhaled NO, or Decreased Control with Inhaled/Systemic Steroid Taper) (Severe Eosinophilic Asthma) (Lancet, 2012) [MEDLINE]
- The Role of Bronchial Biopsy in the Prediction of Response to Biologic Therapy in Severe Uncontrolled Asthma: A Prospective Study. Chest. 2025 Apr;167(4):945-955. doi: 10.1016/j.chest.2024.11.045 [MEDLINE]
- Background: Up to two-thirds of patients with severe uncontrolled asthma (SUA) who received biologic therapy do not have a complete response
- Research question: Can bronchial biopsy (BB) play a role in the identification of patients with SUA who have a better response to biologic therapy?
- Study design and methods: This prospective multicenter study included consecutive patients with SUA who were candidates for biologic therapy. They underwent bronchoscopy and BB prior to biologic therapy, and clinical response was evaluated 6 months later. BB was evaluated according to a previously validated pathological score (PS) and was compared with a score of type 2 (T2) inflammation (T2 score) that includes blood eosinophil count and fractional exhaled nitric oxide in predicting response to biologic therapy. Response was graded as super-response, good response, and partial/no response according to a composite score that includes exacerbations, oral corticosteroid steroid (OCS) use, asthma control test, and improvement in FEV1
- Results: A total of 92 patients were recruited. Of the 92 patients recruited, 78 completed the study. Among them, 63 received an anti-IL-5 or IL-5 receptor (anti-IL5/5R) (mepolizumab, reslizumab, and benralizumab) while 15 received dupilumab. The proportion of super-responders was 36.5% in the anti-IL5/5R group and 26.6% in the dupilumab group (P = .126). The PS was the only variable independently associated with response; the T2 score was not. Super-responders had a statistically significantly higher PS. Response was better predicted by the PS compared with the T2 score in those receiving OCSs and especially in those taking anti-IL5/5Rs. Reduced eosinophil levels (< 10 eosinophils/field) were associated with poor response to biologic therapy
- Interpretation: Our findings indicate that BB is more precise in the prediction of response to biologic therapy than the T2 score, especially in those requiring OCSs or receiving anti-IL5/5Rs. Tissue eosinophilia is the main driver of this predictive capacity. However, other items in the PS related to bronchial remodeling might contribute to the identification of response to biologic therapy.
Reslizumab (Cinqair) (see Reslizumab)
- Indications
- Asthma with Eosinophilic Inflammation (Peripheral Eosinophilia >300/µL)
- FDA Approved for Add-On Maintenance Therapy in Severe Asthma Patients with Eosinophilic Phenotype at Least 18 y/o
- Asthma with Eosinophilic Inflammation (Peripheral Eosinophilia >300/µL)
- Pharmacology: monoclonal antibody against interleukin-5 (IL-5)
- Administration: xxxxx
- Clinical Efficacy
- Phase 2 Trials of Reslizumab in Asthma with Peripheral Eosinophilia (Lancet Resp Med, 2015) [MEDLINE]
- Reslizumab Decreased Asthma Exacerbations
- Adverse Effects: similar to placebo (although 2 patients experienced anaphylaxis)
- Phase 3 Trial of Reslizumab in Poorly-Controlled Asthma (Chest, 2016) [MEDLINE]
- In Overall Population: Reslizumab Did Not Improve FEV1
- In Subgroup of Patients with Baseline Peripheral Blood Eosinophils <400/μL: Reslizumab Did Not Improve FEV1
- In Subgroup of Patients with Baseline Peripheral Blood Eosinophils ≥400/μL: Reslizumab Improved FEV1, ACQ-7, Rescue SABA Use
- In Overall Population: Reslizumab Did Not Improve FEV1
- Phase 2 Trials of Reslizumab in Asthma with Peripheral Eosinophilia (Lancet Resp Med, 2015) [MEDLINE]
- The Role of Bronchial Biopsy in the Prediction of Response to Biologic Therapy in Severe Uncontrolled Asthma: A Prospective Study. Chest. 2025 Apr;167(4):945-955. doi: 10.1016/j.chest.2024.11.045 [MEDLINE]
- Background: Up to two-thirds of patients with severe uncontrolled asthma (SUA) who received biologic therapy do not have a complete response
- Research question: Can bronchial biopsy (BB) play a role in the identification of patients with SUA who have a better response to biologic therapy?
- Study design and methods: This prospective multicenter study included consecutive patients with SUA who were candidates for biologic therapy. They underwent bronchoscopy and BB prior to biologic therapy, and clinical response was evaluated 6 months later. BB was evaluated according to a previously validated pathological score (PS) and was compared with a score of type 2 (T2) inflammation (T2 score) that includes blood eosinophil count and fractional exhaled nitric oxide in predicting response to biologic therapy. Response was graded as super-response, good response, and partial/no response according to a composite score that includes exacerbations, oral corticosteroid steroid (OCS) use, asthma control test, and improvement in FEV1
- Results: A total of 92 patients were recruited. Of the 92 patients recruited, 78 completed the study. Among them, 63 received an anti-IL-5 or IL-5 receptor (anti-IL5/5R) (mepolizumab, reslizumab, and benralizumab) while 15 received dupilumab. The proportion of super-responders was 36.5% in the anti-IL5/5R group and 26.6% in the dupilumab group (P = .126). The PS was the only variable independently associated with response; the T2 score was not. Super-responders had a statistically significantly higher PS. Response was better predicted by the PS compared with the T2 score in those receiving OCSs and especially in those taking anti-IL5/5Rs. Reduced eosinophil levels (< 10 eosinophils/field) were associated with poor response to biologic therapy
- Interpretation: Our findings indicate that BB is more precise in the prediction of response to biologic therapy than the T2 score, especially in those requiring OCSs or receiving anti-IL5/5Rs. Tissue eosinophilia is the main driver of this predictive capacity. However, other items in the PS related to bronchial remodeling might contribute to the identification of response to biologic therapy.
Benralizumab (Fasenra) (see Benralizumab)
- Clinical Efficacy
- The Role of Bronchial Biopsy in the Prediction of Response to Biologic Therapy in Severe Uncontrolled Asthma: A Prospective Study. Chest. 2025 Apr;167(4):945-955. doi: 10.1016/j.chest.2024.11.045 [MEDLINE]
- Background: Up to two-thirds of patients with severe uncontrolled asthma (SUA) who received biologic therapy do not have a complete response
- Research question: Can bronchial biopsy (BB) play a role in the identification of patients with SUA who have a better response to biologic therapy?
- Study design and methods: This prospective multicenter study included consecutive patients with SUA who were candidates for biologic therapy. They underwent bronchoscopy and BB prior to biologic therapy, and clinical response was evaluated 6 months later. BB was evaluated according to a previously validated pathological score (PS) and was compared with a score of type 2 (T2) inflammation (T2 score) that includes blood eosinophil count and fractional exhaled nitric oxide in predicting response to biologic therapy. Response was graded as super-response, good response, and partial/no response according to a composite score that includes exacerbations, oral corticosteroid steroid (OCS) use, asthma control test, and improvement in FEV1
- Results: A total of 92 patients were recruited. Of the 92 patients recruited, 78 completed the study. Among them, 63 received an anti-IL-5 or IL-5 receptor (anti-IL5/5R) (mepolizumab, reslizumab, and benralizumab) while 15 received dupilumab. The proportion of super-responders was 36.5% in the anti-IL5/5R group and 26.6% in the dupilumab group (P = .126). The PS was the only variable independently associated with response; the T2 score was not. Super-responders had a statistically significantly higher PS. Response was better predicted by the PS compared with the T2 score in those receiving OCSs and especially in those taking anti-IL5/5Rs. Reduced eosinophil levels (< 10 eosinophils/field) were associated with poor response to biologic therapy
- Interpretation: Our findings indicate that BB is more precise in the prediction of response to biologic therapy than the T2 score, especially in those requiring OCSs or receiving anti-IL5/5Rs. Tissue eosinophilia is the main driver of this predictive capacity. However, other items in the PS related to bronchial remodeling might contribute to the identification of response to biologic therapy.
Anti-Interleukin-13 (IL-13) Therapy
Lebrikizumab (TNX-650) (see Lebrikizumab)
- Indications
- Asthma with Elevated Periostin Level (see Serum Periostin)
- Pharmacology: IgG4 humanized monoclonal antibody that binds to IL-13, inhibiting IL-13 function
- IL-13 Induces Bronchial Epithelial Cells to Secrete Periostin (a Matricellular Protein that May Induce Airway Remodeling in Asthma)
- Administration: SQ
- Clinical Efficacy
- Randomized, Double-Blinded, Placebo-Controlled Trial of Lebrikizumab in Adult Asthmatics Who Were Uncontrolled Despite Inhaled Corticosteroid Therapy (NEJM, 2011) [MEDLINE]
- Lebrizumab Improved FEV1: lebrikizumab resulted in a larger increase in FEV1 in patients with high pre-treatment periostin levels (8.2% increase in FEV1) vs patients with low with pre-treatment periostin levels (1.6% increase in FEV1) -> therefore, high levels of IL-13 activity predicted lebrikizumab efficacy
- Lebrikizumab Decreased the Fraction of Exhaled Nitric Oxide (FeNO) Indirectly
- Randomized, Double-Blinded, Placebo-Controlled Trial of Lebrikizumab in Adult Asthmatics Who Were Uncontrolled Despite Inhaled Corticosteroid Therapy (NEJM, 2011) [MEDLINE]
Bronchial Thermoplasty (see Bronchial Thermoplasty)
- Technique: use of thermal energy to directly target the airway smooth muscle responsible for bronchoconstriction
- Alair Bronchial Thermoplasty System (Boston Scientific; Marlborough, Massachusetts)
Adverse Effects
- Bronchial Irritation
- Cough (see Cough)
- Discolored Sputum
- Upper Respiratory Infection
- Wheezing
- Worsening of Dyspnea (see Dyspnea)
Clinical Efficacy
- Air Trial in Moderate-Severe Asthma (NEJM, 2007) [MEDLINE]
- Bronchial Thermoplasty Decreased Asthma Exacerbations
- At 12 mo, Bronchial Thermoplasty Improved Morning Peak Expiratory Flow, Scores on AQLQ, ACQ, Percentage of Symptom-Free Days, Symptom Scores, Need for Rescue Medication
- Bronchial Thermoplasty Had No Impact on FEV1
- AIR-2 Trial Studying the Long-Term Safety and Efficacy of Bronchial Thermoplasty in Patients with Severe Persistent Asthma (J Allergy Clin Immunol, 2013) [MEDLINE]
- Bronchial Thermoplasty Has Durable 5-Year Benefits with Regard to Asthma Control (Maintained Decrease in Severe Exacerbations and ED Visits for Respiratory Symptoms) and Safety
- Systematic Review of Bronchial Thermoplasty in Moderate-Severe Asthma (Cochrane Database Syst Rev, 2014) [MEDLINE]
- Bronchial Thermoplasty Provides a Modest Clinical Benefit in Quality of Life and Lower Rates of Asthma Exacerbation
- Bronchial Thermoplasty Provides No Significant Difference in Asthma Control Scores
Yoga (see xxx)
- Clinical Efficacy
- Systematic Review and Meta-Analysis of Yoga in the Treatment of Asthma (Ann Allergy Asthma Immunol, 2014) [MEDLINE]
- Yoga Improved Asthma Control, Asthma Symptoms, Peak Expiratory Flow Rate, FEV1/FVC Ratio, and Quality of Life in Asthma, as Compared to Usual Care
- Systematic Review and Meta-Analysis of Yoga in the Treatment of Asthma (Ann Allergy Asthma Immunol, 2014) [MEDLINE]
Macrolides (see Macrolides)
Agents
- Azithromycin (Zithromax) (see Azithromycin)
- Clarithromycin (Biaxin) (see Clarithromycin)
- Roxithromycin (Biaxsig, Coroxin, Romac, Roxar, Roximycin, Roxl-150, Roxo, Roxomycin, Rulid, Rulide, Surlid, Tirabicin, Xthrocin) (see Roxithromycin)
- Troleandomycin (Triocetin, Tekmisin) (see Troleandomycin)
Clinical Efficacy-Azithromycin (see Azithromycin)
- Cochrane Database Systematic Review of Macrolides in Asthma (Cochrane Database Syst Rev, 2015) [MEDLINE]
- Macrolides Had No Clinical Benefit for Most Clinical Outcomes (Although Evidence was Very Low Quality)
- However, Macrolides May Improve Some Measures of Lung Function
- Macrolides Had No Clinical Benefit for Most Clinical Outcomes (Although Evidence was Very Low Quality)
- Australian AMAZES Trial of Azithromycin in Persistent Uncontrolled Asthma (Lancet, 2017) [MEDLINE]
- In Adults with Persistent Uncontrolled Asthma, Azithromycin (x 48 wks) Decreased the Rate of Asthma Exacerbation and Improved Quality of Life
Metformin (XXX) see Metformin)
Clinical Efficacy
- xxx
- Metformin use is associated with decreased asthma exacerbations in adolescents and young adults. Pediatr Pulmonol. 2023 Sep 29. doi: 10.1002/ppul.26704 [MEDLINE]
- Rationale: Metformin is a commonly used antidiabetes medication with suggested anti-inflammatory and antioxidative effects. Metformin use has been associated with lower risk of asthma exacerbations and hospitalizations in adults. Here, we aimed to evaluate how asthma exacerbation rates changed after adolescents and young adults were prescribed metformin, and to learn if those changes were related to metformin prescription adherence
- Methods: Using secondary data of patients between 12 and 20 years old with asthma diagnosis and a metformin prescription from the Arkansas All Payers Claim Database and Arkansas School body mass index (BMI) database, we estimated the change in annualized asthma exacerbation rates after metformin prescription. We also evaluated the association of prescription adherence to the changes in those rates using univariate and multivariate regression models
- Results: A total of 464 patients met inclusion criteria. Outpatient exacerbation rates decreased after metformin prescription (13.4% only before vs. 7.8% only after, p = .009), and the annualized rate decreased more after metformin prescription as adherence increased (rank r = -.165, p < .001). After adjusting for potential confounders-age, sex, BMI, and inhaled corticoid steroid use-the strength of the association was attenuated
- Conclusions: Asthma exacerbation rates decreased after metformin prescription, but a larger sample of patients who have experienced exacerbations and including patients with asthma who have not been prescribed metformin is needed to better know whether these decreases are driven by metformin use.
Azithromycin (see xxxx)
- xxxx
- Azithromycin induced asthma remission in adults with persistent uncontrolled asthma: a secondary analysis of a randomised, double-blind, placebo-controlled trial. Chest. 2024 Feb 29:S0012-3692(24)00284-8. doi: 10.1016/j.chest.2024.02.048 [MEDLINE]
- Background: Asthma remission is a potential treatment goal
- Research question: Does adding azithromycin to standard therapy in patients with persistent uncontrolled asthma induce remission compared to placebo?
- Study design and methods: This secondary analysis used data from the AMAZES clinical trial – a double-blind placebo-controlled trial that evaluated the safety and efficacy of azithromycin on asthma exacerbations. The primary remission definition (referred to as clinical remission) was zero exacerbations and zero oral corticosteroids (OCS) during the previous six months evaluated at 12 months and Asthma Control Questionnaire (ACQ-5) ≤1 at 12 months. Secondary remission definitions included clinical remission plus lung function criteria (post-bronchodilator FEV1≥80% or post-bronchodilator FEV1≤5% decline from baseline) and complete remission (sputum eosinophils<3% plus the above criteria). Sensitivity analyses explored the robustness of primary and secondary remission definitions. The predictors of clinical remission were identified
- Results: 335 participants (41.5% male; median [Q1, Q3] age 61.01 [51.03, 68.73] years) who completed the 12-month treatment period were included in the analysis. Twelve months treatment with azithromycin induced asthma remission in a subgroup of patients, and a significantly higher proportion in the azithromycin arm achieved both clinical remission (50.6% vs 38.9%; p=0.032) and clinical remission plus lung function criteria (50.8% vs 37.1%; p=0.029) compared with placebo. In addition, a higher proportion of the azithromycin group achieved complete remission (23% vs 13.7%; p=0.058). Sensitivity analyses supported these findings. Baseline factors such as better asthma-related quality of life and absence of OCS burst in the previous year predicted the odds of achieving clinical remission. Azithromycin induced remission in both eosinophilic and noneosinophilic asthma
- Interpretation: Adults with persistent symptomatic asthma achieved a higher remission rate when treated with azithromycin. Remission on treatment may be an achievable treatment target in moderate/severe asthma, and future studies should consider remission as an outcome measure.
Probiotics
Clinical Efficacy
- xxxx
- Immunomodulatory effects of probiotic supplementation in patients with asthma: a randomized, double-blind, placebo-controlled trial. Allergy Asthma Clin Immunol. 2023 Jan 2;19(1):1. doi: 10.1186/s13223-022-00753-4 [MEDLINE]
- The present study was a randomized, double-blind, placebo-controlled trial that enrolled 40 asthmatic patients. They were treated with probiotics or placebo: 1 capsule/day for 8 weeks. Pulmonary function tests, IL-4 and IFN-γ levels, and expression of microRNAs were assessed at baseline and after treatment.
- Results: The results showed that the expression of miR-16, miR146-a and IL-4 levels in patients with asthma after receiving probiotic supplementation was significantly reduced and miR-133b expression was increased. In addition, pulmonary function tests showed a significant improvement in Forced Expiratory Volume in 1 s and Forced Vital Capacity after receiving probiotics
- Conclusion: In our study, 8-week treatment with probiotic supplementation led to reduced Th2 cells-associated IL-4 and improved Forced Expiratory Volume and Forced Vital Capacity. It appears probiotics can be used in addition to common asthma treatments.
Step-Down Therapy
- In the setting of improved control on an inhaled steroid and other agents, stepping down the dose of inhaled steroid is preferred to minimize the potential complications (including adrenal suppression, cataracts, osteoporosis, growth rate
- The Relationship Between Real-World Inhaled Corticosteroid Adherence and Asthma Outcomes: A Multilevel Approach. J Allergy Clin Immunol Pract. 2020 Feb;8(2):626-634. doi: 10.1016/j.jaip.2019.09.003 [MEDLINE]
- Background: Low inhaled corticosteroid (ICS) adherence is associated with increased asthma burden. This relationship is likely bidirectional, and may vary across adherence stages (initiation, implementation, and persistence). Studies rarely examine reciprocal influences
- Objective: To investigate the relationship between ICS implementation and asthma-related outcomes over 2 years, considering bidirectionality and temporal sequence
- Methods: Primary care records (1987-2012) from the Optimum Patient Care Research Database, United Kingdom, were used. Eligible patients were 6 years or older and had 3 or more years of continuous registration starting 1 year before ICS initiation (index date), physician-diagnosed asthma, 2 or more ICS and/or short-acting β-agonist prescriptions each follow-up year, and no long-acting β-agonists, leukotriene receptor antagonists, or maintenance oral corticosteroids in the preceding year. ICS implementation (percentage of days covered) and risk domain asthma control (RDAC; no asthma-related hospitalizations, emergency visits, or outpatient visits and no oral corticosteroid or antibiotic prescriptions with evidence of respiratory review) were estimated for each prescription interval (period between 2 successive prescriptions). Multilevel analyses modeled bidirectional relationships between ICS implementation and RDAC (and its components), controlling for sociodemographic and clinical characteristics
- Results: In prescription data from 10,472 patients, ICS implementation in the preceding interval did not predict RDAC, but was weakly positively associated with simultaneous RDAC. Being male, non-current smoker, without chronic obstructive pulmonary disease diagnosis, and with fewer than 4 comorbidities significantly increased odds of RDAC. Asthma-related antibiotics and outpatient visits in the same interval and short-acting β-agonist overuse in the preceding and same interval predicted lower ICS implementation
- Conclusions: Patients may adapt their ICS use to their current needs without this impacting later RDAC.
Agents/Treatments With Unclear or No Clinical Benefit in the Treatment of Asthma
- Acupuncture (see Acupuncture)
- Clinical Efficacy
- Review of Complementary/Alternative Therapies in the Treatment of Asthma (Respirology, 2006) [MEDLINE]
- No Clinical Benefit Has Been Demonstrated for Acupuncture, Breathing Exercises, Herbal Products, or Homeopathy
- Review of Complementary/Alternative Therapies in the Treatment of Asthma (Respirology, 2006) [MEDLINE]
- Clinical Efficacy
- Anti-Fungal Therapy
- Itraconazole (Sporanox) (see Itraconazole)
- Voriconazole (Vfend) (see Voriconazole)
- Anti-Interleukin-2 Receptor (IL-2R) Antibody
- Anti-Interleukin-4 Receptor Alpha Subunit Antibody
- Anti-Thymic Stromal Lymphopoietin
- Anti-Tumor Necrosis Factor-α (Anti-TNFα) Therapy (see Anti-Tumor Necrosis Factor-α Therapy)
- Etanercept (Enbrel) (see Etanercept)
- Golimumab (Simponi, Simponi Aria) (see Golimumab)
- Breathing Exercises
- Clinical Efficacy
- Systematic Review of Breathing Exercise in Asthma (Cochrane Database Syst Rev, 2013) [MEDLINE]
- Breathing Exercises Had No Clinical Benefit in Asthma
- Systematic Review of Breathing Exercise in Asthma (Cochrane Database Syst Rev, 2013) [MEDLINE]
- Clinical Efficacy
- Calcium Channel Blockers (see Calcium Channel Blockers)
- Chloroquine (see Chloroquine)
- Colchicine (see Colchicine)
- Cyclosporine A (see Cyclosporine A)
- Dapsone (see Dapsone)
- Furosemide (Lasix) (see Furosemide)
- Clinical Efficacy
- XXXX
- Clinical Efficacy
- GATA3-Specific DNAzyme
- Glucocorticoid Receptor Agonist AZD5423
- Gold (see Gold)
- Hydroxychloroquine (Plaquenil) (see Hydroxychloroquine)
- Hypnotherapy (see Hypnotherapy)
- Intravenous Immunoglobulin (IVIG) (see Intravenous Immunoglobulin)
- Ketotifen (Zaditor) (see Ketotifen): no clinical benefit to alter airway reactivity in persistent asthma
- Methotrexate (see Methotrexate
- Nebulized Heparin (see Heparin)
- Neobulized Lidocaine (see Lidocaine)
- Omega-3 Fatty Acids
- Clinical Efficacy
- Trial of Omega-3 Fatty Acids in Asthma (Chest, 2015) [MEDLINE]
- Omega-3 Fatty Acids (x 3 wks) Did Not Improve Airway Hyperresponsiveness, Sputum Eosinophil Counts, or Urinary Mast Cell Mediator Excretion in Moderate Asthma
- Trial of Omega-3 Fatty Acids in Asthma (Chest, 2015) [MEDLINE]
- Clinical Efficacy
- Vitamin C (see Vitamin C)
- Clinical Efficacy
- Systematic Review of Vitamin C in the Treatment of Asthma (Cochrane Database Syst Rev, 2013) [MEDLINE]
- Vitamin C Had No Clinical Benefit in the Management of Asthma or Exercise-Induced Bronchospasm
- Systematic Review of Vitamin C in the Treatment of Asthma (Cochrane Database Syst Rev, 2013) [MEDLINE]
- Clinical Efficacy
- Vitamin D (see Vitamin D)
- Clinical Efficacy
- Multi-Center Vitamin D Add-On Therapy Enhances Corticosteroid Responsiveness in Asthma (VIDA) Trial of Vitamin D3 in the Symptomatic Asthmatics with Vitamin D Deficiency (JAMA, 2014) [MEDLINE]
- Vitamin D3 Did Not Decrease the Rate of First Treatment Failure or Exacerbation in Adults with Persistent Asthma and Vitamin D Deficiency
- Systematic Review and Meta-Analysis of Vitamin D in Childhood Asthma (Nutrients, 2022) [MEDLINE]
- Vitamin D Supplementation Did Not Decrease the Risk of Asthma Exacerbation in Children Overall But May Decrease the Risk of Asthma Exacerbation in Children with Serum 25(OH)D Concentration <10 ng/mL
- Vitamin D Supplementation Decreased the severity of atopic dermatitis and symptoms of allergic rhinitis in children
- Multi-Center Vitamin D Add-On Therapy Enhances Corticosteroid Responsiveness in Asthma (VIDA) Trial of Vitamin D3 in the Symptomatic Asthmatics with Vitamin D Deficiency (JAMA, 2014) [MEDLINE]
- Clinical Efficacy
Management of Specific Asthma Situations
Management of Asthma in the Setting of Metabolic Syndrome/Obesity (see Obesity)
- XXXXX
- Metabolic Medications and Youth Hospitalizations for Asthma: A Pre-Post Quasi-Experimental Study. CHEST Pulm. 2025 Sep;3(3):100185. doi: 10.1016/j.chpulm.2025.100185 [MEDLINE]
- Background: Obesity and metabolic dysregulation can lead to adverse outcomes in people with asthma. We hypothesized that pharmacological treatment of metabolic conditions in youth with asthma is associated with lowered risk of severe asthma exacerbations
- Research question: Is metabolic pharmacotherapy associated with a lower risk of severe asthma exacerbations among children and young adults with metabolic dysregulation?
- Study design and methods: This retrospective, quasi-experimental, longitudinal study examined severe asthma exacerbations (those requiring hospitalization or an emergency department [ED] visit) among youth aged 5-25 years with asthma and a history of a metabolic condition (obesity, diabetes, or hypertension). Definitions and diagnoses were based on documented international classification of diseases (ICD) codes. We compared the odds of severe asthma exacerbations before and after the initiation of metabolic pharmocotheraphy using adjusted piecewise generalized linear mixed models
- Results: The cohort consisted of 783 patients, predominantly female (73.7%), white (71.6%), and non-Hispanic (90.4%). Metformin was the most frequently prescribed metabolic medication (75.4%). Before initiating metabolic pharmocotheraphy, the odds of severe asthma exacerbations increased by 29% per year (OR=1.29, 95%CI = 1.12-1.49). Conversely, following the commencement of metabolic pharmacotherapy, the odds of severe asthma exacerbations decreased by 66% per year (OR=0.34, 95%CI = 0.23-0.50), showing a statistically significant and marked difference between the pre- and post-treatment periods
- Interpretation: Our findings show that the odds of severe asthma excerbations are substantially lower after the initiation of metabolic pharmacotheraphy, highlighting the positive impact that treatment of metabolic syndromes could have in reducing the risk of severe asthma exacerbations. This underscores the interconnectedness of metabolic and respiratory health and the need for further research into effective treatment strategies for individuals with asthma and obesity-related metabolic conditions.
Management of Severe Asthma with Fungal Sensitization (SAFS)
- Azole Antifungals (see xxxx)
- xxxx
- Clinical Efficacy
- FAST Trial (Am J Respir Crit Care Med, 2009) [MEDLINE]
- Oral Antifungal Therapy Improved Quality of Life in about 60% of SAFS Patients
- FAST Trial (Am J Respir Crit Care Med, 2009) [MEDLINE]
Management of Exercise-Induced Asthma
- xxx
Management of Asthma in Pregnancy (see Pregnancy)
- Pregnancy-Related Complications During Acute Asthma Exacerbation
- Preterm Labor: may be treated with magnesium sulfate (this induces bronchodilation and also avoids excessive ß2-agonist use)
- Uterine Contractions: common during acute asthma exacerbations
- Agents
- Albuterol is the Preferred SABA in Pregnancy (see Albuterol, [[Albuterol]]): due to safety considerations
- Inhaled Corticosteroids are the Preferred Long-Term Controller Medication in Pregnancy (see Corticosteroids)
- Budesonide (see Budesonide): preferred inhaled corticosteroid, due to the amount of safety data available
–Asthma Drugs: safe in pregnancy (steroids increase cleft palate in animals/ only effect in humans is a decrease birth weight by 300-400 g)
Management of Asthma in the Perioperative Period
- General Information
- Asthma Increases the Risk of Peri-Operative Complications
- Bronchospasm Triggered by Intubation
- Hypoxemia/Hypercapnia
- Impairment of Cough and Secretion Clearance
- Reaction to Latex and Anesthetics: in cases where sensitivity to these has been previously documented
- Respiratory Infection
- Asthma Increases the Risk of Peri-Operative Complications
- Management
- Optimize Asthma Control Prior to Surgery: may require short course of systemic corticosteroids
- In Asthmatic Patients Who Have Received Oral Systemic Corticosteroids Within 6 Months Prior to Surgery, Stress-Dose Corticosteroids (Hydrocortisone 100 mg q8hrs) Should Be Considered Peri-Operatively: subsequently, corticosteroids should be tapered within 24 hrs after surgery
Management of Asthma Risks with Scuba Diving
- Asthma may increase the risk of diving-related complications
- Spirometry does not predict risk
- Wheezing induced by cold, emotion, or exercise are probable contraindications to diving
- Clearance for Diving: normal PFT s + normal exercise test (to 80% maximal HR)
- Prior to testing, no short-acting ß2-agonists x 8 hrs, no long-acting ß2 agonists x 48 hrs, and no ipratropium x 24 hrs
- Normal Test: <15% decrease in FEV1 with exercise)
