Epidemiology
Risk Factors
Immune Defect
- Agammaglobulinemia (see xxxx): B-cell defect
- Ataxia-Telangiectasia
- Chediak-Higashi Syndrome (see Chediak-Higashi Syndrome)
- Chronic Granulomatous Disease (CGD) (see Chronic Granulomatous Disease)
- Chronic Lymphocytic Leukemia (CLL) (see Chronic Lymphocytic Leukemia): B-cell defect
- C3 Complement Deficiency (see xxxx)
- Dysglobulinemia: B-cell defect
- Hyper IgE-Recurrent Infection Syndrome (see Hyper IgE-Recurrent Infection Syndrome)
- IL-1 Receptor-Associated Kinase (IRAK4) Defect
- Leukocyte Adhesion Deficiency (see Leukocyte Adhesion Deficiency)
- Multiple Myeloma (see Multiple Myeloma): B-cell defect
- Neutropenia (see Neutropenia)
- Protein-Calorie Malnutrition (see Protein-Calorie Malnutrition)
- Severe Combined Immunodeficiency (SCID) (see Severe Combined Immunodeficiency)
Implantable Device
- Artificial Joint
- Automatic Implantable Cardiodefibrillator (AICD) (see Automatic Implantable Cardiodefibrillator)
- Cardiac Pacemaker (see Cardiac Pacemaker)
- Heart Valve
Other
- Cystic Fibrosis (CF) (see Cystic Fibrosis)
Microbiology
Staphylococcus Aureus is a Member of Staphylococcus Genus (see Staphylococcus)
- Staphylococcus Aureus is a Gram-Positive Bacterium
- Like Other Staphylococci, Staphylococcus Aureus Appears Microscopically as Grape-Like Clusters
Methicillin-Resistant Staphylococcus Aureus (MRSA)
Definitions
- By Definition, All MRSA Isolates Carry the mecA Gene or the mecC Gene
- These Genes Confer Resistance to All β-Lactam Antibiotics (Including Cephalosporins and Carbapenems)
Risk Factors for Methicillin-Resistant Staphylococcus Aureus (MRSA)
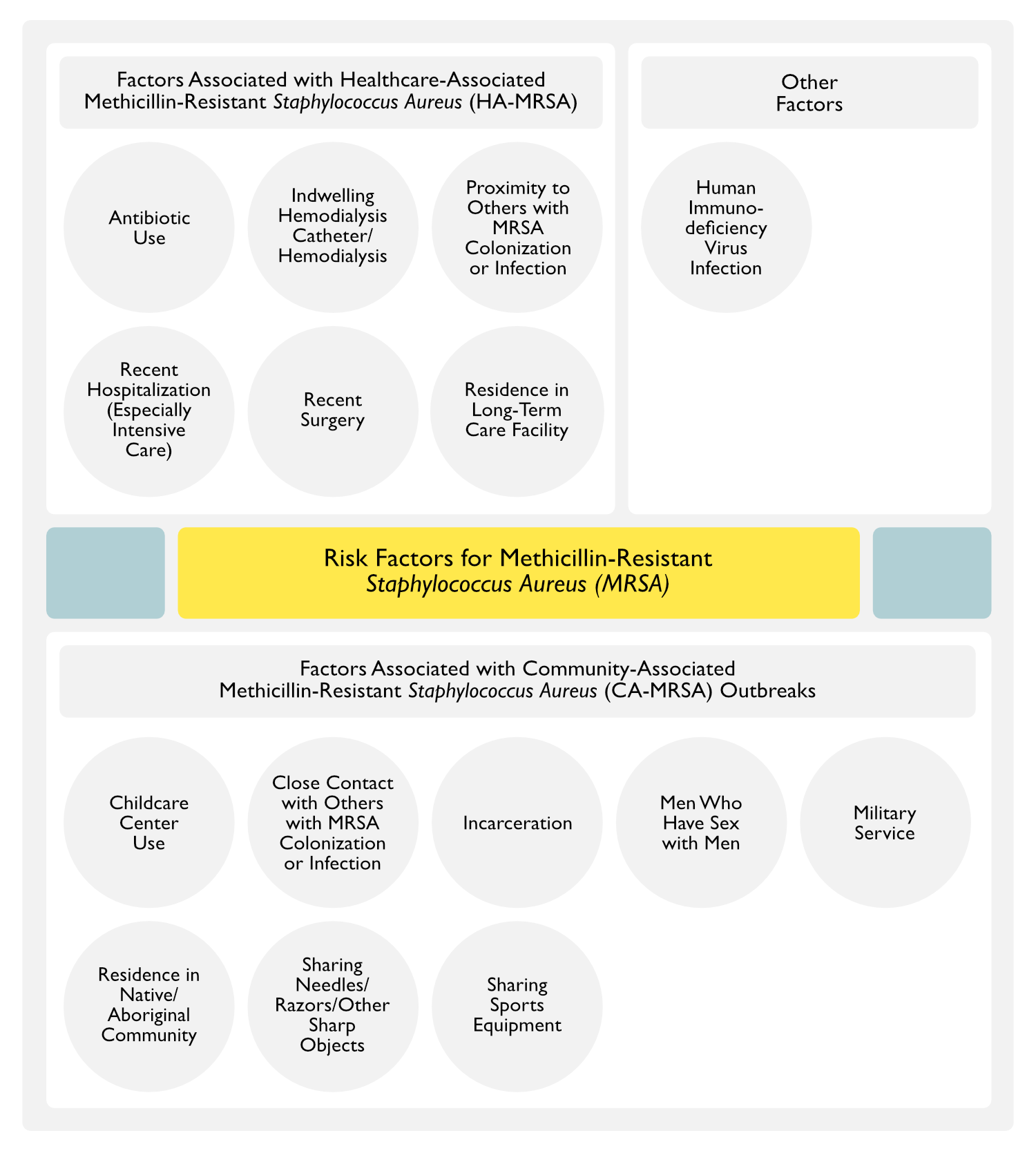
- Factors Associated with Healthcare-Associated MRSA (HA-MRSA)
- Antibiotic Use
- Related to Selective Pressure of the Antibiotic (Highest Risk is Associated with Cephalosporin and Fluoroquinolone Use) (Emerg Infect Dis, 2007) [MEDLINE] (J Antimicrob Chemother, 1998) [MEDLINE] (Infect Control Hosp Epidemiol, 1996) [MEDLINE]
- In a UK Case-Control Study, the Odds Ratio of MRSA Diagnosis for Patients Who were Prescribed 1, 2-3, or ≥4 antimicrobial drugs were 1.57 (95% CI: 1.36-1.80), 2.46 (CI: 2.15-2.83), and 6.24 (95% CI: 5.43-7.17), Respectively (Emerg Infect Dis, 2007) [MEDLINE]
- In a UK Case-Control Study, the Risk for Community-Acquired MRSA Increased with the Number of Antimicrobial Drug Prescriptions, Appeared to Vary According to Antimicrobial Drug Classes Prescribed During the Previous Year, and was Highest for Fluoroquinolone (Odds Ratio 3.37; CI: 2.80-4.09) and Macrolides (Odds Ratio 2.50; CI: 2.14-2.91)
- Related to Selective Pressure of the Antibiotic (Highest Risk is Associated with Cephalosporin and Fluoroquinolone Use) (Emerg Infect Dis, 2007) [MEDLINE] (J Antimicrob Chemother, 1998) [MEDLINE] (Infect Control Hosp Epidemiol, 1996) [MEDLINE]
- Indwelling Hemodialysis Catheter/Hemodialysis (see Hemodialysis) (MMWR Morb Mortal Wkly Rep, 2007) [MEDLINE]
- Proximity to Others with MRSA Colonization or Infection (Ann Intern Med, 1982) [MEDLINE] (Infect Dis Clin North Am, 1989) [MEDLINE] (Clin Infect Dis, 2004) [MEDLINE]
- Recent Hospitalization (Especially Intensive Care) (Ann Intern Med, 1982) [MEDLINE] (Infect Dis Clin North Am, 1989) [MEDLINE] (Clin Infect Dis, 2004) [MEDLINE]
- Recent Surgery
- Residence in Long-Term Care Facility
- Healthcare-Associated MRSA (HA-MRSA) is Prevalent in Long-Term Care Facility Residents (and They are Frequently Transferred Between Hospitals and Long-Term Care Facilities, Facilitating the Spread of MRSA) (Infect Control Hosp Epidemiol, 1995) [MEDLINE]
- Antibiotic Use
- Factors Associated with Community-Associated MRSA (CA-MRSA) Outbreaks
- Childcare Center Use
- Close Contact with Others with MRSA Colonization or Infection
- Incarceration
- Men Who Have Sex with Men
- Military Service
- Residence in Native/Aboriginal Community
- Sharing Needles (Injection Drug Abuse)/Razors/Tattooing/Body Shaving/Other Sharp Objects (see Injection Drug Abuse)
- Sharing Sports Equipment
- Other Factors
- Human Immunodeficiency Virus (HIV) Infection (see Human Immunodeficiency Virus)
- HIV Infection Increases the Risk of MRSA Colonization and Infection
- Risk Factors for MRSA Infection in the Setting of HIV Infection (J Acquir Immune Defic Syndr, 2005) [MEDLINE]
- Advanced Immunosuppression (CD4 Count <50 cells/microL)
- High Plasma HIV RNA (>100,000 Copies/microL)
- Lack of Antiretroviral Therapy
- Human Immunodeficiency Virus (HIV) Infection (see Human Immunodeficiency Virus)
Healthcare-Associated Methicillin-Resistant Staphylococcus Aureus (HA-MRSA) vs Community-Associated Methicillin-Resistant Staphylococcus Aureus (CA-MRSA) Infection
- General Comments
- Patients Can Develop MRSA Colonization in the Hospital and Manifest Infection While in the Community
- Alternately, Patients Can Develop MRSA Colonization in the Community and Then Manifest Infection in the Hospital
- In a Review of 352 Patients with HA-MRSA, the Percentage of Isolates with CA-MRSA Molecular Typing Increased from 17% to 56% Between 1999-2004 (Emerg Infect Dis, 2007) [MEDLINE]
- Healthcare-Associated Methicillin-Resistant Staphylococcus Aureus (HA-MRSA) Infection: HA-MRSA is defined as an MRSA infection which occurs >48 hrs following hospitalization (hospital-onset HA-MRSA) or an MRSA infection which occurs outside of the hospital within 12 mos of exposure to health care such as a history of surgery/hospitalization/dialysis/residence in a long-term care facility (community-onset HA-MRSA) (JAMA, 2007) [MEDLINE]
- Epidemiology
- MRSA is the Leading Etiology of Surgical Site Infections in Both Community and Tertiary Hospitals
- Since the 1960’s, HA-MRSA Has Been a Problem in Hospital Settings with a Progressive Increase in the Prevalence of Antimicrobial Resistance in Hospital-Acquired Staphylococcus Aureus Infections
- In a US Study of Over 24,000 Cases of Hospital-Acquired Staphylococcus Aureus Bloodstream Infections, MRSA Isolates Increased from 22% to 57% Between 1995 and 2001 (Clin Infect Dis, 2004) [MEDLINE]
- In a VA Study Employing Multifaceted Infection Control Measures Between 2005-2017, There was a 43% Decline in Staphylococcus Aureus Infections (Accounted for Mostly by a 55% Decrease in MRSA Infections) (MMWR Morb Mortal Wkly Rep, 2019) [MEDLINE]
- Risk Factors for HA-MRSA Infection (Ann Intern Med, 1982) [MEDLINE] (Infect Dis Clin North Am, 1989) [MEDLINE] (Clin Infect Dis, 2004) [MEDLINE]
- Antibiotic Use
- Hemodialysis
- Intensive Care
- MRSA Colonization
- Prolonged Hospitalization
- Proximity to Others with MRSA Colonization or Infection
- Risk Factors for Post-Discharge MRSA Infection (Clin Infect Dis, 2016) [MEDLINE]
- Discharge to Nursing Home
- Discharge with a Central Venous Catheter/Other Invasive Device
- MRSA Colonization
- Presence of a Chronic Wound
- Microbiology
- HA-MRSA Strains Tend to Possess Multidrug Resistance and Carry Staphylococcal Cassette Chromosome (SCCmec) Type II (JAMA, 2003) [MEDLINE]
- HA-MRSA Strains Generally Demonstrate a USA100 or USA200 Pulse-Field Electrophoresis (PFGE) Pattern
- Clinical
- HA-MRSA is Associated with Severe, Invasive Disease (Such as Skin/Soft Tissue Infection, Bloodstream Infection, and Pneumonia) and is Routinely Implicated in Nearly Every Type of Hospital-Acquired Infection (Ventilator-Associated Pneumonia, Skin/Soft Tissue Infection, etc)
- Due to Propensity of MRSA to Form Biofilms on Invasive Devices (Such as Endotracheal Tubes, Urinary Catheters, and Intravascular Catheters) (Clin Infect Dis, 2007) [MEDLINE]
- Biofilms Facilitate MRSA Survival and Multiplication on Surfaces, Prolonging the Duration of Organism Exposure to Antibiotics and Facilitating the Potential Transfer of Antibiotic Resistance Genes Between Organisms
- HA-MRSA is Associated with Severe, Invasive Disease (Such as Skin/Soft Tissue Infection, Bloodstream Infection, and Pneumonia) and is Routinely Implicated in Nearly Every Type of Hospital-Acquired Infection (Ventilator-Associated Pneumonia, Skin/Soft Tissue Infection, etc)
- Prognosis
- Patients with MRSA Infection Have Higher Mortality, Longer Hospital Stays, and Higher Health Care Costs, as Compared to Patients with MSSA Infection (Arch Intern Med, 2002) [MEDLINE]
- Epidemiology
- Community-Associated Methicillin-Resistant Staphylococcus Aureus (CA-MRSA) Infection: defined as an MRSA infection which occurs in the absence of healthcare exposure (JAMA, 2007) [MEDLINE]
- Epidemiology
- CA-MRSA was Initially Reported in Injection Drug Users in the Early 1980’s, But Has Since Become the Most Common Etiology of Skin/Soft Tissue Infections Presenting to US Emergency Departments and Ambulatory Clinics
- USA300 Appears to Be More Transmissible Among Household Members than Other CA-MRSA Types
- Community Outbreaks Have Been Reported in Multiple Settings (Including Native/Aboriginal Communities, Sports Teams, Childcare Centers, Military Personnel, Meen Who Have Sex with Men, and Prison Inmates and Guards) (JAMA, 2001) [MEDLINE] (NEJM, 2005;352(5):468 [MEDLINE] (Ann Intern Med, 2008) [MEDLINE] (Clin Infect Dis, 2009) [MEDLINE]
- Animals Have Also Been Identified as a Source of CA-MRSA Transmission
- However, Many Patients with CA-MRSA Have No Identifiable Risk Factors (Clin Infect Dis, 2007) [MEDLINE]
- Risk Factors for CA-MRSA (with Relatively Poor Predictive Value for CA-MRSA) (J Infect Dis, 1998) [MEDLINE] (JAMA, 1998) [MEDLINE] (MMWR Morb Mortal Wkly Rep, 2001) [MEDLINE] (JAMA, 2001) [MEDLINE] (JAMA, 2003) [MEDLINE] (MMWR Morb Mortal Wkly Rep, 2003) [MEDLINE] (J Clin Microbiol, 2004) [MEDLINE] (Clin Infect Dis, 2004) [MEDLINE] (NEJM, 2005) [MEDLINE] (NEJM, 2005) [MEDLINE] (Lancet Infect Dis, 2006) [MEDLINE] (MMWR Morb Mortal Wkly Rep, 2006) [MEDLINE] (Clin Infect Dis, 2015) [MEDLINE] (MMWR Morb Mortal Wkly Rep, 2018) [MEDLINE]
- Close Contact with Others with MRSA Colonization or Infection
- Cosmetic Body Shaving
- Incarceration
- Sharing Equipment Which is Not Cleaned Between Users
- Skin Trauma (Abrasions, Lacerations, Tattoos, Injection Drug Abuse)
- Microbiology
- CA-MRSA Strains Frequently Carry the SCCmec Type IV or V and Frequently Carry Genes for the Panton-Valentine Leukocidin (PVL) Cytotoxin Which Confers Enhanced Virulence (Lancet, 2006) (Antimicrob Agents Chemother, 2002) [MEDLINE] ( Lancet, 2002) [MEDLINE] [MEDLINE] (J Clin Microbiol, 2006) [MEDLINE] (J Infect Dis, 2006) [MEDLINE] (Ann Intern Med, 2006) [MEDLINE]
- CA-MRSA Strains Generally Demonstrate a USA300 or USA400 Pulse-Field Electrophoresis (PFGE) Pattern
- Clinical
- CA-MRSA is Most Commonly Associated with Skin and Soft Tissue Infections in Young Healthy Patients (NEJM, 2005) [MEDLINE]
- CA-MRSA Has Also Been Associated with Necrotizing Pneumonia, Osteomyelitis, Urinary Tract Infection, Infective Endocarditis, and Sepsis (with or without Waterhouse-Friderichsen Syndrome)
- Treatment
- Most CA-MRSA Strains are Sensitive to Non-β-Lactam Antibiotics
- Epidemiology
Physiology
Staphylococcus Aureus Colonization (Carriage)
Sites of Staphylococcus Aureus Colonization (Eur J Clin Microbiol Infect Dis, 2001) [MEDLINE] (J Clin Microbiol, 2006) [MEDLINE] (Diagn Microbiol Infect Dis, 2008) [MEDLINE]
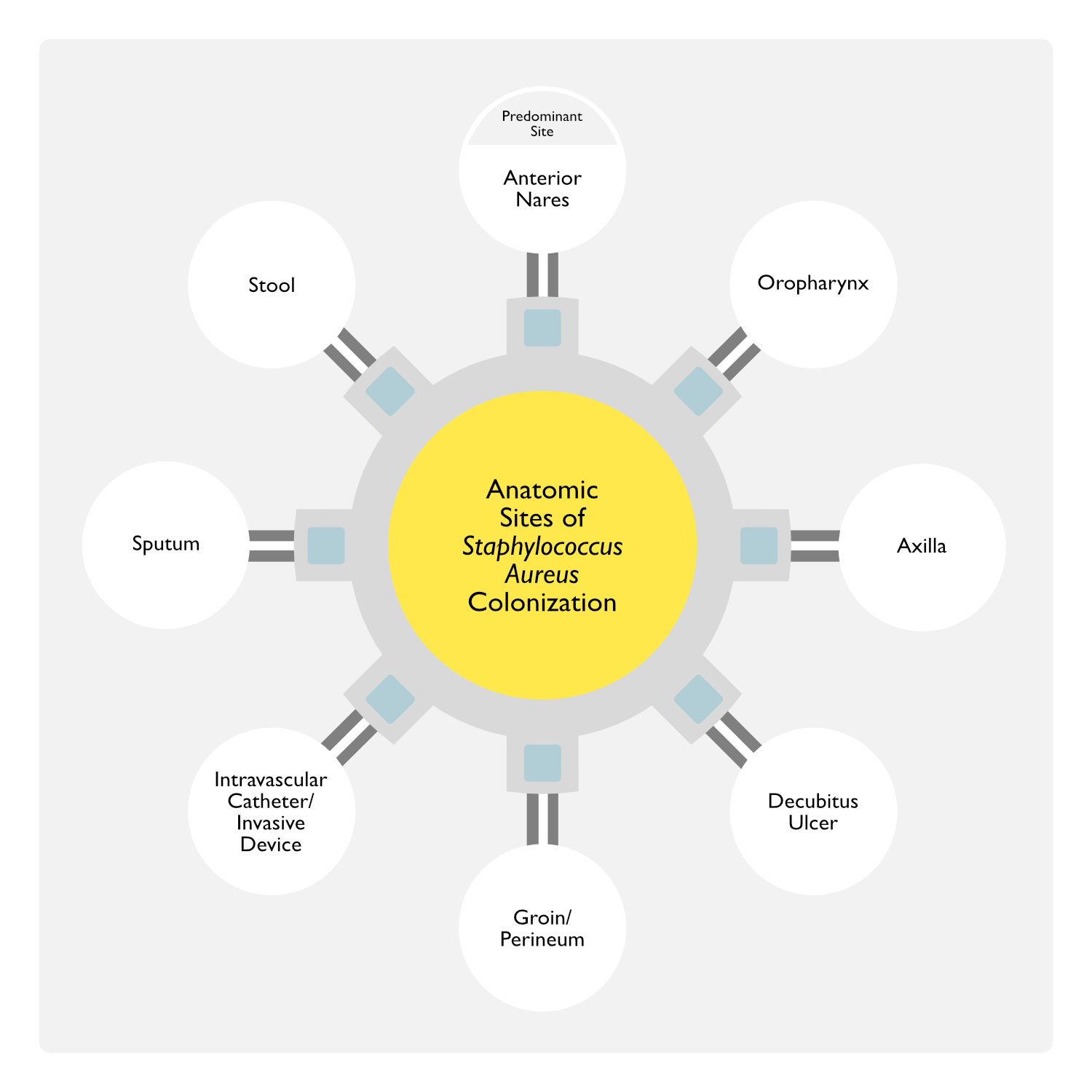
- Anterior Nares
- Anterior Nares are the Primary Site of Colonization
- A Majority of Individuals with Nasal Colonization are Also Colonized on Other Areas of Intact Skin (Such as the Hands, Axillae, Perineum, and in the Umbilicus in Infants)
- Other Anatomic Sites
- General Comments
- These Other Non-Nasal Sites (Oropharynx, Axilla, etc) Have Lower Sensitivity for the Detection of Staphylococcus Aureus Possibly Due to Lower Staphylococcus Aureus Colonization Rates or Higher Quantities of Competing Microbiologic Flora at these Sites
- Oropharynx
- Some Studies Suggest that Oropharyngeal Colonization May Occur without Nasal Colonization (Thereby Being Missed on Routine Nasal Screening)
- Swiss Study of Sites of Staphylococcus Aureus Colonization (n = 3,464) (Arch Intern Med, 2009) [MEDLINE]: 48% of Patients were Staphylococcus Aureus Nasal Carriers, While 12% of Patients were Exclusive Staphylococcus Aureus Throat Carriers
- Some Studies Suggest that Oropharyngeal Colonization May Occur without Nasal Colonization (Thereby Being Missed on Routine Nasal Screening)
- Axilla
- Decubitus Ulcer (see Decubitus Ulcer)
- Genitourinary Tract
- Groin/Perineum
- Intravascular Catheter (Central Venous Catheter, Arterial Line, etc)/Invasive Device
- Sputum
- Stool
- General Comments
Incidence of Nasal Methicillin-Resistant Staphylococcus Aureus (MRSA) Colonization
- Study Using Data from the National Health and Nutrition Examination Survey (NHANES) in the Early 2000’s (J Infect Dis, 2008) [MEDLINE]
- Prevalence of Colonization with Staphylococcus Aureus Decreased from 32.4% in 2001-2002 to 28.6% in 2003-2004 (p < 0.01)
- Prevalence of Colonization with Methicillin-Resistant Staphylococcus Aureus (MRSA) Increased from 0.8% to 1.5% (p < 0.05)
- Risk Factors for MRSA Colonization in Males
- Healthcare Exposure
- Risk Factors for MRSA Colonization in Females
- Having Been Born in the US
- Age ≥60 y/o
- Diabetes Mellitus
- Poverty
- In 2003-2004, 19.7% (95% CI: 12.4%-28.8%) of MRSA-Colonized Persons Carried a Pulse-Field Gel Electrophoresis (PFGE) Pattern Associated with Community Transmission (i.e. CA-MRSA)
- Data Suggest that Community-Associated (CA-MRSA) May Cause Disease in the Absence of Prior Nasal Colonization and/or that CA-MRSA Favors Other Sites of Colonization (Skin, Throat, Gastrointestinal Tract, etc)
- Nasal MRSA Colonization is Present in Approximately 7% of Hospitalized Patients in the US (Am J Infect Control, 2012) [MEDLINE]
- Nasal MRSA Colonization is Present in Approximately 4-15% of Healthcare Workers (Ann Emerg Med, 2008) [MEDLINE] (Ann Emerg Med, 2008) [MEDLINE]
Duration of Methicillin-Resistant Staphylococcus Aureus (MRSA) Colonization (Infect Control Hosp Epidemiol, 2006) [MEDLINE] (Arch Intern Med, 2009) [MEDLINE] (BMC Infect Dis, 2014) [MEDLINE] (Am J Infect Control, 2014) [MEDLINE] (Clin Infect Dis, 2015) [MEDLINE]
- Previously Nasal MRSA Colonized Patients Should Be Considered to Be at Risk for Continued Nasal MRSA Carriage for at Least 4 Years (Clin Infect Dis, 2009) [MEDLINE]
- During the First Year After the Positive Nasal MRSA Culture Result was Obtained, 48.8% of the Patients (95% CI: 45.8%-51.7%) Remained Colonized
- At 4 Years, 21.2% of the Patients (95% CI: 13.1%-31.4%) Remained Colonized
- In Other Studies, the Median Time to Nasal MRSA Clearance Has Been Reported to Be Around 88 wks (BMC Infect Dis, 2014) [MEDLINE]
- Tobacco Smoke Exposure Increases the Degree of Nasal Staphylococcus Aureus Colonization
- In Experimental Models of Nasal Inoculation, Smokers Had Higher Nasal Staphylococcus Aureus Organism Loads than Non-Smokers (Infect Immun, 2018) [MEDLINE]
- Smoking Cessation Improved Clearance of Staphylococcus Aureus Nasal Colonization
- Tobacco Smoke Enhanced Staphylococcus Aureus Biofilm Formation (Sci Rep, 2019) [MEDLINE]
- In Experimental Models of Nasal Inoculation, Smokers Had Higher Nasal Staphylococcus Aureus Organism Loads than Non-Smokers (Infect Immun, 2018) [MEDLINE]
Staphylococcus Aureus Colonization Increases the Risk of Subsequent Staphylococcus Aureus Infection
- In a Study of Hospitalized Patients (n = 758), Nasal MRSA Colonization Present at the Time of Admission Increased the Risk for MRSA Infection within 1 Year, as Compared to No Staphylococcal Colonization (Relative Risk 9.5; 95% CI: 3.6-25) (Clin Infect Dis, 2004) [MEDLINE]
- Acquisition of Nasal MRSA Colonization During Hosptalization Also Increased the Risk for Subsequent MRSA Infection within 1 Year, as Compared to No Acquisition (Relative Risk 12; 95% CI: 4.0-38)
- In the National Nosocomial Infections Surveillance (NNIS) System, Baseline Nasal MRSA Colonization Increased the Risk for MRSA Infection (19%) in the Year Following Hospital Admission, as Compared to 2% in Those without Baseline Nasal MRSA Colonization (Am J Infect Control, 2004) [MEDLINE]
- In a Large VA Study of MRSA Carriage Rates and MRSA Infection Rates in Hospitalized Patients, MRSA Colonization Significantly Increased the Risk of Subsequent MRSA Infection (Approximatly 60% of MRSA Infections Occurred After Discharge from the Hospital (Clin Infect Dis, 2019) [MEDLINE]
- Because MRSA Colonization Increases the Risk for MRSA Infection Following Invasive Medical/Surgical Procedures, Preoperative Testing for Nasal MRSA Colonization is Common
Staphylococcus Aureus Transmission
- Reservoirs for Staphylococcus Aureus Transmission
- Individuals Colonized with Methicillin-Resistant Staphylococcus Aureus (MRSA)
- Methicillin-Resistant Staphylococcus Aureus (MRSA)-Contaminated Surfaces
- Modes of Staphylococcus Aureus Transmission and Colonization (J Clin Pathol, 1961) [MEDLINE] (Eur J Clin Microbiol Infect Dis, 1994) [MEDLINE]
- Via the Contaminated Hands of Healthcare Workers
- Most Common Mode of Transmission for Healthcare-Associated MRSA (HA-MRSA)
- Via Contact with Contaminated Wounds or Dressings of Infected Patients
- Via Contact with Another Individual’s Colonized Intact Skin
- Via Contact with Contaminated Inanimate Objects/Fomites (Razors, Towels, Environmental Surfaces, etc)
- Contaminated Environmental Surfaces are a Common Source for Healthcare-Associated MRSA (HA-MRSA)
- Contaminated Inanimate Objects/Fomites are a Common Source for Community-Associated MRSA (CA-MRSA)
- Via Inhalation of Aerosolized Droplets from Chronic Nasal Carriers
- Via the Contaminated Hands of Healthcare Workers
- Transmission of Healthcare-Associated MRSA (HA-MRSA) to Household Contacts
- Study of Transmission of Healthcare-Associated MRSA (HA-MRSA) to Household Contacts of Patients Who are Colonized on Hospital Discharge (Arch Intern Med, 2009) [MEDLINE]: n = 148 patients discharged to home healthcare
- 75% of Colonized Patients Cleared the HA-MRSA within 1 yr (Estimated Median Time to Clearance of 282 Days) (95% CI: 233-313 days)
- Clearance of MRSA was Associated with Self-Sufficiency in Daily Activities (Hazard Ratio 0.63; 95% CI: 0.40-1.00; P = 0.049)
- Of the 188 included household contacts, 36 acquired MRSA (19.1%)
- Transmission Occurred in Nearly 20% of Household Contacts
- Factors Associated with Household MRSA Acquisition
- Older Age (Adjusted Odds Ratio 1.71 Per Life Decade; 95% CI: 1.32-2.21; P = 0.001)
- Participation in the Health Care of the Index Patient (Adjusted Odds Ratio 3.58; 95% CI: 1.33-9.62; P = 0.01)
- Study of Transmission of Healthcare-Associated MRSA (HA-MRSA) to Household Contacts of Patients Who are Colonized on Hospital Discharge (Arch Intern Med, 2009) [MEDLINE]: n = 148 patients discharged to home healthcare
Risk Factors for Nosocomial Staphylococcus Aureus Bacteremia
- MRSA Colonization
- Staph aureus bacteremia occurs almost exclusively in patients with previous nasal colonization
- Up to 25% of patients admitted to the hospital will become nasal Staph aureus carriers (often with prevalent hospital strains)
- This rate may climb to 60% in those with ESRD, diabetes, and HIV
- MRSA carriers have 3.9x the rate of Staph aureus bacteremia than MSSA carriers
- Relative risk was 0.04 for patients receiving antibiotics (especially beta-lactams and glycopeptides), as compared to those not receiving antibiotics
- MSSA nasal carriage is predominantly community-acquired and is more prevalent than MRSA nasal carriage
- Most cases of Staph aureus bacteremia are related to line infection
(mechanical ventilation, underlying malignancy, and severity of illness at the time of ICU admission (by SAPS score) are not risk factors for Staph aureus bacteremia)
Diagnosis
- Sputum Gram Stain/Culture and Sensitivity
- MRSA: recent community-acquired MRSA strains have been found to cause human disease (Daum, 2002; Frank, 2002) — Unlike institutional strains of MRSA, these typically are sensitive to vanco, clinda, tetracyclines, aminoglycosides, fluoroquinolones, bactrim, and erythro
- Vanco-Intermediate Sensitivity Staph aureus (VISA): present in US since 2002/refractory to vanco therapy
- Vanco-Resistant Staph aureus (VRSA): cases reported in US in 2002
- Linezolid-Resistant Staph aureus: reported in one case in 2001
Nasal Methicillin-Resistant Staphylococcus Aureus (MRSA) Testing (see Nasal Methicillin-Resistant Staphylococcus Aureus Testing)
Clinical Efficacy
- Meta-Analysis of Methicillin-Resistant Staphylococcus Aureus (MRSA) Nasal Screening to Rule Out MRSA Pneumonia (Clin Infect Dis, 2018) [MEDLINE]: n = 1,563 (22 studies)
- For All MRSA Pneumonia Types
- Pooled Sensitivity of MRSA Nare Testing for All MRSA Pneumonia Types: 70.9%
- Pooled Specificity of MRSA Nare Testing for All MRSA Pneumonia Types: 90.3%
- With a 10% Prevalence of Potential MRSA Pneumonia, the Calculated Positive Predictive Value was 44.8% and the Negative Predictive Value was 96.5%
- For Community-Acquired Pneumonia (CAP) and Healthcare-Associated MRSA Pneumonia (HCAP)
- Pooled Sensitivity of MRSA Nare Testing for All MRSA Pneumonia Types: 85%
- Pooled Specificity of MRSA Nare Testing for All MRSA Pneumonia Types: 92.1%
- With a 10% Prevalence of Potential MRSA Pneumonia, the Calculated Positive Predictive Value was 56.8% and the Negative Predictive Value was 98.1%
- For Ventilator-Associated MRSA Pneumonia
- Pooled Sensitivity of MRSA Nare Testing for All MRSA Pneumonia Types: 40.3%
- Pooled Specificity of MRSA Nare Testing for All MRSA Pneumonia Types: 93.7%
- With a 10% Prevalence of Potential MRSA Pneumonia, the Calculated Positive Predictive Value was 35.7% and the Negative Predictive Value was 94.8%
- For All MRSA Pneumonia Types
- Data Supporting the Negative Predictive Value of Rapid Methicillin-Resistant Staphylococcus Aureus (MRSA) Testing are Robust (American Thoracic Society and Infectious Diseases Society of America 2019 Clinical Practice Guidelines for the Diagnosis and Treatment of Adults with Community-Acquired Pneumonia) (Am J Respir Crit Care Med, 2019) [MEDLINE]
- Treatment for Methicillin-Resistant Staphylococcus Aureus (MRSA) Pneumonia Can Generally Be Withheld When the Nasal Methicillin-Resistant Staphylococcus Aureus (MRSA) Swab is Negative, Especially in Non-Severe Community-Acquired Pneumonia
- However, the Positive Predictive Value of Methicillin-Resistant Staphylococcus Aureus (MRSA) Culture is Not as High
- Therefore, When the Nasal Methicillin-Resistant Staphylococcus Aureus (MRSA) Swab is Positive, Coverage for Methicillin-Resistant Staphylococcus Aureus (MRSA) Pneumonia Should Generally Be Initiated (In the Specific Clinical Situations Noted Below), But Blood/Sputum Cultures Should Be Obtained and Therapy Deescalated if Cultures are Negative
Clinical Manifestations
Cardiovascular Manifestations
Automatic Implantable Cardioverter-Defibrillator (AICD) or Cardiac Pacemaker Infection (see Automatic Implantable Cardioverter-Defibrillator and Cardiac Pacemaker)
- Epidemiology
- In Patients with a Cardiac Device Who Have Staphylococcus Aureus Bacteremia, Rate of Device Infection Can Be as High as 45%
- Mechanism of Infection
- Contamination During Implantation
- Hematogenous Seeding (via Bacteremia)
Endocarditis (see Endocarditis)
- Epidemiology
- Staphylococcus Aureus is the Most Common Etiology of Infective Endocarditis in Resource-Rich Settings (JAMA, 2005) [MEDLINE] (Eur J Clin Microbiol Infect Dis, 2016) [MEDLINE] (Open Forum Infect Dis, 2021) [MEDLINE]
- Incidence of Endocarditis in the Setting of Staphylococcus Aureus Bacteremia is About 10-15% (J Am Coll Cardiol, 1997) [MEDLINE] (J Clin Oncol, 2000) [MEDLINE] (Arch Intern Med, 2003) [MEDLINE] (Medicine-Baltimore, 2003) [MEDLINE] (Am Heart J, 2004) [MEDLINE] (J Heart Valve Dis, 2005) [MEDLINE] (JAMA, 2014) [MEDLINE] (Am J Med, 2005) [MEDLINE]
- Risk Factors for Infective Endocarditis in the Setting of Staphylococcus Aureus Bacteremia (Ann Intern Med, 1993) [MEDLINE] (J Clin Oncol, 2000) [MEDLINE] (Mayo Clin Proc, 2007) [MEDLINE] (PLoS One, 2015) [MEDLINE]
- Bacteremia of Unclear Origin
- Intravascular Catheter Infection
- Intravenous Drug Abuse (IVDA)
- Persistent Bacteremia
- Pre-Existing Cardiac Abnormality
- Prosthetic Heart Valve
- Infective Endocarditis Due to Staphylococcus Aureus is More Severe Than Infective Endocarditis Due to Other Organisms (Heart, 2005) [MEDLINE]
- Staphylococcus Aureus Infective Endocarditis Has a Higher Incidence of Sepsis
- Staphylococcus Aureus Infective Endocarditis Has a Higher Incidence of Major Neurologic Events
- Staphylococcus Aureus Infective Endocarditis Has a Higher Incidence of Multi-Organ Failure
- Staphylococcus Aureus Infective Endocarditis Has a Higher Mortality Rate: 34% (vs 10% with other organisms)
- Mechanism of Infection
- Hematogenous Seeding (Via Bacteremia)
Dermatologic Manifestations
Cellulitis (see Cellulitis)
- Epidemiology
- Mechanism of Infection
- Skin Breach
Erysipelas (see Erysipelas)
- Mechanism of Infection
- Skin Breach
Folliculitis
- Mechanism of Infection
- Skin Breach
Hidradenitis Suppurativa
- Mechanism of Infection
- Skin Breach
Impetigo (see Impetigo)
- Mechanism of Infection
- Skin Breach
Mastitis (see Mastitis)
- Mechanism of Infection
Purpura Fulminans (see Purpura Fulminans)
- Mechanism
Scarlet Fever (see Scarlet Fever)
- Epidemiology: less common etiology of scarlet fever than Streptococcus Pyogenes
- Mechanism:
Skin Abscess (see Skin Abscess)
- Epidemiology
- Mechanism of Infection
- Skin Breach
Staphylococcal Scalded Skin Syndrome (Ritter Disease) (see Staphylococcal Scalded Skin Syndrome)
- Mechanism of Infection
- Skin Breach
Surgical Site Infection
- Epidemiology
- Mechanism of Infection
- Skin Breach
- Clinical
- Superficial Skin/Soft Tissue Infections Caused by Staphylococcus Aureus Usually Present with Purulence or Abscess
Gastroenterologic Manifestations
Staphylococcus Aureus Toxinosis
- Mechanism
- Ingestion of Food-Borne Toxin
- Clinical
- Diarrhea
Hematologic Manifestations
Hemolytic Anemia (see Hemolytic Anemia)
- Physiology
- Due to Mechanical Red Blood Cell Destruction
Splenic Abscess (see Splenic Abscess)
- Mechanism of Infection
- Hematogenous Seeding (Via Bacteremia)
Neurologic Manifestations
Intracranial Epidural Abscess (see Intracranial Epidural Abscess)
- Epidemiology
- Usually Results from Neurosurgical Intervention
- Mechanism of Infection
- XXXXX
Meningitis (see Meningitis)
- Mechanisms of Infection
- Neurosurgical Intervention: most common mechanism
- Hematogenous Seeding (Via Bacteremia): less common mechanism
Spinal Epidural Abscess (see Spinal Epidural Abscess)
- Mechanisms of Infection
- Hematogenous Seeding (Via Bacteremia)
- Direct Extension from Contiguous Source
Otolaryngologic Manifestations
Acute Hospital-Acquired Bacterial Rhinosinusitis (see Acute Rhinosinusitis)
- Epidemiology –XXXX
Pulmonary Manifestations
Acute Respiratory Distress Syndrome (ARDS) (see Acute Respiratory Distress Syndrome)
- Epidemiology
- XXXX
Bronchiolitis Obliterans (see Bronchiolitis Obliterans)
- Epidemiology
- Cases Have Been Reported in Patients with Influenza and Staphylococcus Aureus Co-Infection (Arch Bronconeumol, 2017) [MEDLINE]
Interstitial Pneumonia (see Interstitial Lung Disease)
- Epidemiology
- XXXX
- Clinical
- Chest X-Ray (CXR) (see Chest X-Ray)
- Miliary Pattern
- Chest X-Ray (CXR) (see Chest X-Ray)
Necrotizing Pneumonia (see Necrotizing Pneumonia and Pulmonary Gangrene)
- Epidemiology
- Community-Associated MRSA (CA-MRSA) Infection is Often Associated with Severe Necrotizing Pneumonia (Clin Infect Dis, 2002) [MEDLINE] (Lancet, 2002) [MEDLINE] (Clin Infect Dis, 2005) [MEDLINE] (Emerg Infect Dis, 2006) [MEDLINE] (Clin Infect Dis, 2007) [MEDLINE] (MMWR Morb Mortal Wkly Rep, 2007) [MEDLINE] (Clin Infect Dis, 2008) [MEDLINE] (Chest, 2010) [MEDLINE]
- Physiology
- The Tendency to Produce Necrotizing Pneumonia Has Been Suggested to Be Mediated Via by the Panton-Valentine Leukocidin (PVL) Toxin, Which is Typically Present in Community-Associated MRSA (CA-MRSA) Strains (Lancet, 2002) [MEDLINE] (Clin Infect Dis, 2005) [MEDLINE] (Emerg Infect Dis, 2006) [MEDLINE] (MMWR Morb Mortal Wkly Rep, 2007) [MEDLINE]
- Diagnosis
- Leukopenia (see Leukopenia)
- Associated with Rapidly Progressive, Severe Disease
- Chest X-Ray (CXR) (see Chest X-Ray)
- XXX
- Chest CT (see Chest Computed Tomography)
- XXXX
- Leukopenia (see Leukopenia)
Necrotizing Tracheobronchitis (see Acute Bronchitis)
- Epidemiology
- Cases of Necrotizing Tracheobronchitis Have Been Reported in the Setting of Influenza and Staphylococcus Aureus Co-Infection (see Influenza Virus) (Int J Legal Med, 2005) [MEDLINE] (Tuberc Respir Dis (Seoul), 2015) [MEDLINE] (J Bronchology Interv Pulmonol, 2017) [MEDLINE] (Infection, 2018) [MEDLINE]
Pneumatocele (see Cystic-Cavitary Lung Lesions)
- Epidemiology
- Occurs Mainly in Childhood Cases
- Diagnosis
- Chest X-Ray (CXR) (see Chest X-Ray)
- Chest CT (see Chest Computed Tomography)
Community-Acquired Pneumonia (CAP) (see Community-Acquired Pneumonia)
- Epidemiology
- EMERGEncy ID NET Study of Staphylococcus Aureus Community-Acquired Pneumonia in the Emergency Department Setting (Clin Infect Dis, 2012) [MEDLINE]: n = 627 patients with community-acquired pneumonia from 12 university-affiliated emergency departments during the winter-spring of 2006 and 2007
- A Pathogen was Identified in 17% of Cases
- Staphylococcus Aureus was Isolated in 4% of All Cases
- MSSA: 2% of All Cases
- MRSA: 2.4% (Range: 0-5%) of All Cases
- MRSA was Isolated in 5% of Cases Admitted to the Intensive Care Unit
- Features Associated with MRSA Isolation
- Patient History of MRSA
- Nursing Home Admission in the Previous Year
- Close Contact in the Previous Month with Someone with a Skin Infection
- Multiple Infiltrates or Cavities on Chest Radiograph
- Comatose State
- Death in the Emergency Department
- Multi-Country Global Initiative for Methicillin-Resistant Staphylococcus Aureus Pneumonia (GLIMP) Study in Community-Dwelling Adults (Requiring Hospitalization) (Lancet Infect Dis, 2016) [MEDLINE]: n = 3193 community-dwelling adult patients hospitalized for pneumonia
- MRSA Accounted for Approximately 3% of Community-Acquired Pneumonias (Requiring Hospitalization) in Community-Dwelling Adults
- Prevalence Varied Across Countries in the Study
- Risk Factors Associated with MRSA Pneumonia
- Previous MRSA Infection/Colonization (Odds Ratio 6.21; 95% CI: 3.25-11.85)
- Recurrent Skin Infections (2.87, ; 95% CI: 1.10-7.45)
- Severe Pneumonia (2.39, ; 95% CI: 1.55-3.68)
- Among All Staphylococcus Aureus Isolates, 49% were MSSA and 51% were MRSA
- MRSA Accounted for Approximately 3% of Community-Acquired Pneumonias (Requiring Hospitalization) in Community-Dwelling Adults
- Vanderbilt Study of Staphylococcus Aureus Community-Acquired Pneumonia (Requiring Hospitalization) in Adults (Clin Infect Dis, 2016) [MEDLINE]: n = 2259 hospitalized adults with community-acquired pneumonia
- Staphylococcus Aureus Accounted for 1.6% of Community-Acquired Pneumonias (Requiring Hospitalization) in Adults
- MSSA: 1.0%
- MRSA: 0.7%
- Streptococcus Pneumoniae Accounted for 5.1% of Community-Acquired Pneumonias (Requiring Hospitalization) in Adults
- Vancomycin/Linezolid was Administered to 29.8% of Patients within the First 3 Days of Hospitalization
- Chronic Hemodialysis Use was More Common Among Patients with MRSA (20.0%) than Pneumococcal (2.6%) and All-Cause Non-Staphylococcus Aureus (3.7%) CAP
- Otherwise, Clinical Features at Admission were Similar (Including Concurrent Influenza Infection, Hemoptysis, Multilobar Infiltrates, and Prehospital Antibiotics)
- Patients with MRSA CAP Had More Severe Clinical Outcomes Than Those with Pneumococcal CAP, Including Intensive Care Unit Admission (86.7% vs 34.8%) and Inpatient Mortality (13.3% vs 4.4%)
- Staphylococcus Aureus Accounted for 1.6% of Community-Acquired Pneumonias (Requiring Hospitalization) in Adults
- Community-Acquired Staphylococcus Aureus Pneumonia is Usually a Post-Influenza Pneumonia (see Influenza Virus) (Clin Infect Dis, 2005) [MEDLINE] (Emerg Infect Dis, 2006) [MEDLINE] (MMWR Morb Mortal Wkly Rep, 2007) [MEDLINE] (Ann Emerg Med, 2009) [MEDLINE] (Infect Dis Clin North Am, 2013) [MEDLINE]
- However, Most Post-Influenza Pneumonias are Due to Streptococcus Pneumoniae (see Streptococcus Pneumoniae)
- EMERGEncy ID NET Study of Staphylococcus Aureus Community-Acquired Pneumonia in the Emergency Department Setting (Clin Infect Dis, 2012) [MEDLINE]: n = 627 patients with community-acquired pneumonia from 12 university-affiliated emergency departments during the winter-spring of 2006 and 2007
- Mechanisms of Infection
- Seeding of Airways From Staphylococcus Aureus Colonization of Nares/Skin
- Particularly Occurs in Hospitalized Patients Due to Intubation or Other Respiratory Tract Procedures
- Right-Sided Endocarditis with Septic Emboli to Lungs
- Seeding of Airways From Staphylococcus Aureus Colonization of Nares/Skin
- Clinical
- Clinical Case
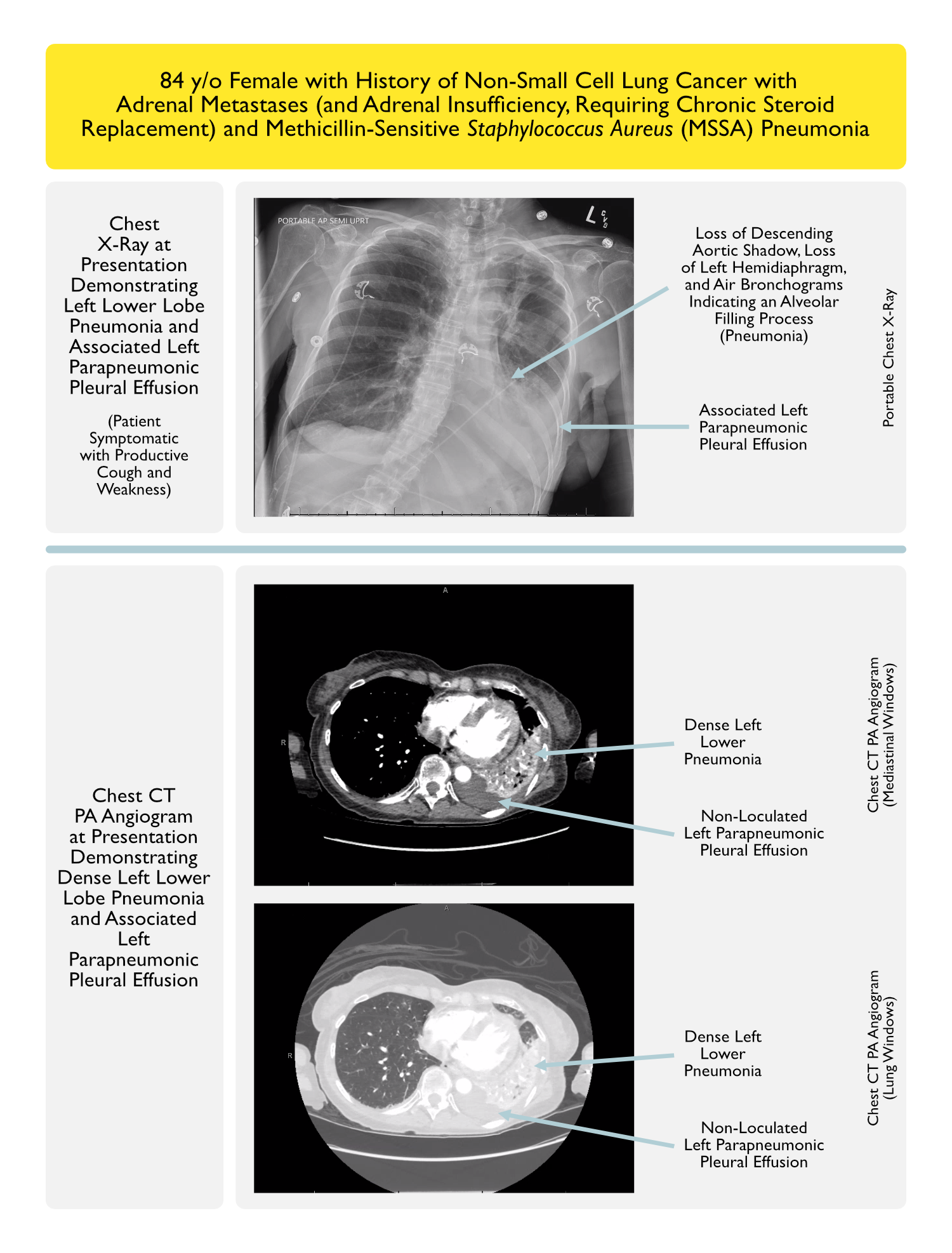
- Prognosis
Tracheobronchitis (see Acute Bronchitis)
- Epidemiology
- Staphylococcus Aureus Tracheobronchitis Has Been Described in Patients with Acute COPD Exacerbation (J Antimicrob Chemother, 1995) [MEDLINE] (Curr Med Res Opin, 2007) [MEDLINE] (see Chronic Obstructive Pulmonary Disease)
- Staphylococcus Aureus Tracheobronchitis May Occur Following Airway Instrumentation (Tracheostomy, Endotracheal Intubation) or Associated
- MRSA Tracheobronchitis Has Been Reported in a Patient with Tracheobronchomalacia (Respiration, 2010) [MEDLINE]
- Staphylococcus Aureus Tracheobronchitis Has Been Reported in an Immunocompromised Patient on Infliximab (Klin Padiatr, 2015) [MEDLINE]
- Clinical
- XXXXXXX
Renal Manifestations
Acute Interstitial Nephritis (see Acute Interstitial Nephritis)
- Epidemiology
- XXXX
Staphylococcus Aureus Bacteriuria (see Urinary Tract Infection)
- Epidemiology
- XXXX
- Mechanisms of Infection
- Ascending Infection Due to Foley Catheter
- Hematogenous Seeding (via Bacteremia)
- Clinical
- In Presence of Foley Catheter: in absence of systemic signs of infection, work-up for bacteremia is not necessary
- In Absence of Foley Catheter: may be indicative of bacteremia, therefore, work-up for bacteremia is required
Reproductive Manifestations
Postpartum Endometritis (see Postpartum Endometritis)
- Epidemiology
- XXXX
- Clinical
- XXXXX
Rheumatologic/Orthopedic Manifestations
Necrotizing Fasciitis (see Necrotizing Fasciitis)
- Mechanism of Infection
- XXXX
Osteomyelitis (see Osteomyelitis)
- Mechanisms of Infection
- Hematogenous Seeding (via Bacteremia): usually vertebral osteomyelitis (possibly with associated Discitis or spinal epidural abscess)
- Direct Extension from Contiguous Source
Prosthetic Septic Arthritis (see Septic Arthritis)
- Mechanisms of Infection
- Early Infection (<3 mo after Surgery):
- Delayed Infection (3-12 mo After Surgery): usually due to acquisition during implantation
- Late Infection (>12 mo After Surgery): usually due to hematogenous seeding of joint (via bacteremia)
Pyomyositis (see Pyomyositis)
- Mechanism of Infection
- Hematogenous Seeding (via Bacteremia)
Septic Arthritis (see Septic Arthritis)
- Mechanism of Infection
- Direct Inoculation of Joint During Joint Surgery
- Extension of Bone Infection Into Joint Space
- Hematogenous Seeding (via Bacteremia): most common mechanism
- Joint Space Synovium is Highly Vascular But Lacks a Basement Membrane
- Joint Trauma
Septic Bursitis (see Septic Bursitis)
- Epidemiology
- XXXX
- Clinical
- XXXXX
Vascular Manifestations
Bacteremia (see Bacteremia)
- Epidemiology
- Staphylococcus Aureus Bacteremia is Defined as a Blood Culture Positive for Staphylococcus Aureus
- Staphylococcus Aureus is a Common Etiology of Both Community-Acquired and Hospital-Acquired Bacteremia
- Physiologic Mechanisms (NEJM, 1998) [MEDLINE] (Clin Infect Dis, 2005) [MEDLINE]
- Primary Staphylococcus Aureus Infection (of Skin/Soft Tissue/Bone/Joint/Pneumonia/Vascular Catheter) with Subsequent Bacteremia
- Staphylococcus Aureus Bacteremia with Seeding of a Previously Sterile Site (Heart Valve/Prosthetic Device/Spinal Epidural Space)
- Clinical
- Fever (see Fever): commonly present
- Hypotension/Sepsis (see Hypotension and Sepsis): commonly present
Intravascular Catheter Infection
- Epidemiology
- Arterial Line (see Arterial Line)
- Central Venous Catheter (CVC) (see Central Venous Catheter)
- Clinical
- May Be Associated with Bacteremia (See Above)
Thrombophlebitis (see Thrombophlebitis)
- Clinical
- Erythema/Pain at Site of Catheter
- Cord
Other Manifestations
Staphylococcal Toxic Shock Syndrome (see Staphylococcal Toxic Shock Syndrome)
- xxxxx
Waterhouse-Friderichsen Syndrome (see Waterhouse-Friderichsen Syndrome)
- xxx
Henoch-Schonlein Purpura (HSP) (see Henoch-Schonlein Purpura)
- Mechanism
- May Be Due to Staphylococcus Aureus Superantigen Action on T-Cells
Prevention
Prevention of Methicillin-Resistant Staphylococcus Aureus (MRSA) Infection in the ICU
- Agents
- Chlorhexidine Gluconate (see Chlorhexidine Gluconate)
- Mupirocin (Bactroban) (see Mupirocin)
- Clinical Efficacy
- Randomized Evaluation of Decolonization versus Universal Clearance to Eliminate MRSA (REDUCE MRSA) Trial (from CDC Prevention Epicenters Program and the AHRQ DECIDE Network and Healthcare-Associated Infections Program) of Universal vs Targeted Decolonization to Prevent Infections in the ICU (NEJM, 2013) [MEDLINE]
- Universal Decolonization (Daily Chlorhexidine Bathing + Nasal Mupirocin BID) was the Most Effective Strategy to Prevent the Rate of MRSA Isolates and Bloodstream Infection from Any Pathogen
- Targeted Decolonization of MRSA Carriers (Daily Chlorhexidine Bathing + Nasal Mupirocin BID) was the Second Most Effective Strategy to Prevent the Rate of MRSA Isolates and Bloodstream Infection from Any Pathogen
- MRSA Screening and Isolation of MRSA Carriers was the Least Effective Strategy to Prevent the Rate of MRSA Isolates and Bloodstream Infection from Any Pathogen: this strategy did not decrease the rate of any infections
- Universal Decolonization Prevented 1 Bloodstream Infection Per Each 54 Patients
- Adverse Events, Which Occurred in 7 Patients, Were Mild and Related to Chlorhexidine
- Trial of Body Surface Decolonization on Bacteriuria and Candiduria in the ICU (Lancet Infect Dis, 2016) [MEDLINE]
- Universal Decolonization (Chlorhexidine) and Short-Course Nasal Mupirocin Significantly Decreased Candiduria and Any Bacteriuria, But Not for Women
- Randomized Evaluation of Decolonization versus Universal Clearance to Eliminate MRSA (REDUCE MRSA) Trial (from CDC Prevention Epicenters Program and the AHRQ DECIDE Network and Healthcare-Associated Infections Program) of Universal vs Targeted Decolonization to Prevent Infections in the ICU (NEJM, 2013) [MEDLINE]
Treatment of Methicillin-Sensitive Staphylococcus Aureus (MSSA)
Anti-Staphylococcal (Penicillinase-Resistant) Penicillins (see Penicillins)
Other Penicillins (see Penicillins)
- Piperacillin-Tazobactam (Zosyn) (see Piperacillin-Tazobactam)
Carbapenems (see Carbapenems)
Cephalosporins (see Cephalosporins)
Fluoroquinolones (see Fluoroquinolones)
- Levofloxacin (Levaquin) (see Levofloxacin)
Treatment of Methicillin-Resistant Staphylococcus Aureus (MRSA)
Uncomplicated Methicillin-Resistant Staphylococcus Aureus (MRSA) Bacteremia
Recommendations (Infectious Diseases Society of America 2011 Guidelines for the Treatment of MRSA) (Clin Infect Dis, 2011) [MEDLINE]
- Definition
- Positive Blood Cultures for MRSA
- Exclusion of Endocarditis
- No Implanted Prosthetics
- Follow-Up Negative Blood Cultures 2-4 Days After Initial Set
- No Metastatic Foci of Infection
- Defervescence within 72 hrs of Initiating Effective Therapy
- Agents
- Vancomycin IV (see Vancomycin): 2 wk course (A-II Recommendation)
- Daptomycin (Cubicin) IV (see Daptomycin): 6 mg/kg/IV qday x 2 wk course (A-I Recommendation)
Complicated Methicillin-Resistant Staphylococcus Aureus (MRSA) Bacteremia
Recommendations (Infectious Diseases Society of America 2011 Guidelines for the Treatment of MRSA) (Clin Infect Dis. 2011) [MEDLINE]
- Definition
- Patients with Positive Blood Cultures for MRSA Who Do Not Meet the Criteria for Uncomplicated Bacteremia
- Agents
- Vancomycin IV (see Vancomycin): 4-6 wk course
- Daptomycin (Cubicin) IV (see Daptomycin): 6 mg/kg/IV qday x 4-6 wk course
- Some Experts Recommend 8-10 mg/kg/IV qday x 4-6 wk course (B-III Recommendation)
Methicillin-Resistant Staphylococcus Aureus (MRSA) Native Valve Infective Endocarditis (see Endocarditis)
Recommendations (Infectious Diseases Society of America 2011 Guidelines for the Treatment of MRSA) (Clin Infect Dis, 2011) [MEDLINE]
- Agents
- Vancomycin IV (see Vancomycin): 6 wk course (A-II Recommendation)
- Daptomycin (Cubicin) IV (see Daptomycin): 6 mg/kg/IV qday x 6 wk course (A-I Recommendation)
- Some Experts Recommend 8-10 mg/kg/IV qday x 6 wk course (B-III Recommendation)
- Other Recommendations
- Addition of Gentamicin to Vancomycin is Not Recommended for the Treatment of MRSA Bacteremia or Native Valve Endocarditis (A-II Recommendation)
- Addition of Rifampin to Vancomycin is Not Recommended for the Treatment of MRSA Bacteremia or Native Valve Endocarditis (A-I Recommendation)
- Additional Blood Cultures 2-4 Days After Initial Positive Set Should Be Obtained to Document Clearance of Bacteremia (A-II Recommendation)
- Echocardiogram (Transesophageal Preferred Over Transthoracic Echocardiogram) is Recommended for All Patients with MRSA Bacteremia (A-III Recommendation)
- Evaluation for Valve Replacement is Recommended for Large Vegetation (>10 mm Diameter), Occurrence or ≥1 Embolic Event During the First 2 Weeks of Therapy, Severe Valvular Insufficiency, or Valvular Perforation or Dehiscence Decompensated Heart Failure, Perivalvular or Myocardial Abscess, New Heart Block, or Persistent Fevers or Bacteremia (A-II Recommendation)
Methicillin-Resistant Staphylococcus Aureus (MRSA) Prosthetic Valve Infective Endocarditis (see Endocarditis)
Recommendations (Infectious Diseases Society of America 2011 Guidelines for the Treatment of MRSA) (Clin Infect Dis, 2011) [MEDLINE]
- Agents
- Combination Vancomycin IV + Rifampin PO/IV 300 mg q8hrs x 6 wks, with Gentamicin 1 mg/kg IV q8hrs x 2 wks (B-III Recommendation) (see Vancomycin, Rifampin, and Gentamicin)
- Other Recommendations
- Early Evaluation for Valve Replacement Surgery is Recommended
Community-Acquired Methicillin-Resistant Staphylococcus Aureus (CA-MRSA) Skin and Soft Tissue Infections (SSTI)
Recommendations (Infectious Diseases Society of America 2011 Guidelines for the Treatment of MRSA) (Clin Infect Dis, 2011) [MEDLINE]
Outpatient Skin and Soft Tissue Infections (SSTI)
- Simple Skin Abscess/Boil (see Skin Abscess): incision and drainage is the primary treatment (A-II Recommendation)
- Antibiotic Therapy is Recommended for Skin Abscess with Any of the Following Characteristics (A-III Recommendation)
- Abscess in an Area that is Difficult to Drain: such as face, hand, genitalia
- Associated Comorbidities or Immunosuppression
- Associated Septic Phlebitis
- Extremes of Age
- Lack of Response to Incision and Drainage
- Rapid Progression in the Presence of Associated Cellulitis
- Severe or Extensive Disease (Multiple Sites of Infection)
- Symptoms/Signs of Systemic Illness
- Antibiotic Therapy is Recommended for Skin Abscess with Any of the Following Characteristics (A-III Recommendation)
- Outpatient Purulent Cellulitis (Cellulitis Associated with Purulent Drainage/Exudate in the Absence of a Drainable Abscess): antibiotic therapy directed against CA-MRSA is recommended -> 5-10 day course (should be individualized based on patient’s clinical response) (A-II Recommendation)
- Empiric Therapy Against β-Hemolytic Streptococci is Likely to Be Unnecessary
- Agents Directed Against CA-MRSA
- Clindamycin PO (see Clindamycin) (A-II Recommendation)
- Doxycycline/Minocycline PO (see Doxycycline or Minocycline) (A-II Recommendation)
- Linezolid (Zyvox) PO (see Linezolid) (A-II Recommendation)
- Sulfamethoxazole-Trimethoprim (Bactrim, Septra) PO (see Sulfamethoxazole-Trimethoprim) (A-II Recommendation)
- Outpatient Non-Purulent Cellulitis (Cellulitis with No Purulent Drainage/Exudate in the Absence of a Drainable Abscess): antibiotic therapy directed against β-hemolytic streptococci is recommended for -> 5-10 day course (should be individualized based on patient’s clinical response) (A-II Recommendation)
- The Role of CA-MRSA Coverage in This Setting is Unknown
- Empiric CA-MRSA Coverage is Recommended for Patients Who Do Not Respond to β-Lactam Therapy and Those with Systemic Toxicity
- Agents Directed Against Both CA-MRSA and β-Hemolytic Streptococci
- Clindamycin PO (see Clindamycin) (A-II Recommendation)
- Combination Doxycycline/Minocycline PO + β-Lactam (Amoxicillin) PO (see Doxycycline or Minocycline, and Amoxicillin) (A-II Recommendation)
- Linezolid (Zyvox) PO (see Linezolid) (A-II Recommendation)
- Sulfamethoxazole-Trimethoprim (Bactrim, Septra) PO (see Sulfamethoxazole-Trimethoprim) (A-II Recommendation)
- Other Recommendations
- Culture from Abscess or Other Purulent SSTI is Recommended in Patients Treated with Antibiotic Therapy, Patients with Severe Local Infection, Patients with Signs of Systemic Toxicity, Patients Who Have Not Responded Adequately to Initial Treatment, or if there is a Concern for Cluster or Outbreak (A-III Recommendation)
- Use of Rifampin Alone or as an Adjunct for the Treatment of Skin and Soft Tissue Infection is Not Recommended (A-III Recommendation)
Inpatient Complicated Skin and Soft Tissue Infections (cSSTI)
- Definition
- Cellulitis
- Deeper Soft Tissue Infection
- Infected Ulcers/Burns
- Major Abscess
- Surgical/Traumatic Wound Infection
- Agents
- Vancomycin IV (see Vancomycin) (A-I Recommendation)
- Dalbavancin (Dalvance) (see Dalbavancin)
- Daptomycin (Cubicin) (see Daptomycin): 4 mg/kg/dose IV qday x 7-14 days (A-I Recommendation)
- Linezolid (Zyvox) IV/PO (see Linezolid): 600 mg BID x 7-14 days (A-I Recommendation)
- Tedizolid (Sivextro) (see Tedizolid)
- Telavancin (Vibativ) IV (see Telavancin): 10 mg/kg/dose qday x 7-14 days (A-I Recommendation)
- Clindamycin PO/IV (see Clindamycin): 600 mg TID x 7-14 days (A-III Recommendation)
- Other Recommendations
- Surgical Debridement and Broad-Spectrum Antibiotics are Recommended
- Culture from Abscess or Other Purulent SSTI is Recommended in Patients Treated with Antibiotic Therapy, Patients with Severe Local Infection, Patients with Signs of Systemic Toxicity, Patients Who Have Not Responded Adequately to Initial Treatment, or if there is a Concern for Cluster or Outbreak (A-III Recommendation)
Methicillin-Resistant Staphylococcus Aureus (MRSA) Community-Acquired Pneumonia (CAP) (see Community-Acquired Pneumonia)
Clinical Efficacy
- VA Retrospective Multicenter Cohort Study of the Impact of Empiric Anti-MRSA Antibiotic Therapy (within the First Day of Hospitalization) in Patients Hospitalized for Community-Acquired Pneumonia (CAP) (JAMA Intern Med, 2020) [MEDLINE]: n = 88, 605 hospitalized patients (from 2008-2013)
- VA Study: population consisted of predominantly males (86 ,851 out of 88,605), median age 70 years (interquartile range: 62-81 y/o)
- Subgroup Analysis was Performed in Patients with Initial Intensive Care Unit Admission, MRSA Risk Factors, Positive Results of a MRSA Surveillance Test, and/or Positive Results of an MRSA Admission Culture
- Empirical anti-MRSA therapy plus standard therapy was significantly associated with an increased adjusted risk of death (adjusted risk ratio [aRR], 1.4 [95% CI, 1.3-1.5]), kidney injury (aRR, 1.4 [95% CI, 1.3-1.5]), and secondary C difficile infections (aRR, 1.6 [95% CI, 1.3-1.9]), vancomycin-resistant Enterococcus spp infections (aRR, 1.6 [95% CI, 1.0-2.3]), and secondary gram-negative rod infections (aRR, 1.5 [95% CI, 1.2-1.8])
- Empirical Anti-MRSA Antibiotic Therapy was Not Associated with Decreased 30-Day Mortality for Any Group of Patients Hospitalized for Pneumonia
Recommendations (Infectious Diseases Society of America 2011 Guidelines for the Treatment of MRSA) (Clin Infect Dis, 2011) [MEDLINE]
- Recommended Agents
- Vancomycin IV (see Vancomycin) (A-II Recommendation)
- Linezolid (Zyvox) IV/PO (see Linezolid): 600 mg BID (A-II Recommendation)
- Clindamycin IV/PO (see Clindamycin): 600 mg TID (B-III Recommendation)
- May Be Used if the Strain is Susceptible
Recommendations for Empiric Antibiotic Therapy for Community-Acquired Pneumonia (American Thoracic Society and Infectious Diseases Society of America 2019 Clinical Practice Guidelines for the Diagnosis and Treatment of Adults with Community-Acquired Pneumonia) (Am J Respir Crit Care Med, 2019) [MEDLINE]


- Outpatient Adult with Risk Factors for Methicillin-Resistant Staphylococcus Aureus (MRSA) (Prior Respiratory Isolation of Methicillin-Resistant Staphylococcus Aureus or Recent Hospitalization with Intravenous Antibiotics in the Last 90 Days)
- Monotherapy with Respiratory Fluoroquinolone (Strong Recommendation, Moderate Quality of Evidence)
- Gemifloxacin (see Levofloxacin): 750 mg PO qday
- Moxifloxacin (see Moxifloxacin): 400 mg PO qday
- Combination Therapy with β-Lactam and Macrolide (or Doxycycline) (Strong Recommendation, Moderate Quality of Evidence for Combination Therapy with Macrolides; Conditional Recommendation, Low Quality of Evidence for Combination Therapy with Doxycycline)
- Amoxicillin-Clavulanic Acid (see Amoxicillin-Clavulanic Acid) or Cefuroxime (see Cefuroxime) or Cefpodoxime (see Cefpodoxime) and Macrolide (see Macrolides) or Doxycycline (see Doxycycline)
- Use Macrolides Only in Regions with Streptococcus Pneumoniae Resistance (MIC ≥16 μg/mL) to Macrolides <25%
- Amoxicillin-Clavulanic Acid (see Amoxicillin-Clavulanic Acid): 500 mg/125 mg PO TID or 875 mg/125 mg PO BID, or 2,000 mg/125 mg PO BID
- Cefuroxime (see Cefuroxime) : 500 mg PO BID
- Cefpodoxime (see Cefpodoxime): 200 mg PO BID
- Azithromycin (see Azithromycin): 500 mg on first day, then 250 mg PO qday
- Clarithromycin (see Clarithromycin): 500 mg PO BID (or extended-release 1,000 mg PO qday)
- Doxycycline (see Doxycycline): 100 mg PO BID
- Amoxicillin-Clavulanic Acid (see Amoxicillin-Clavulanic Acid) or Cefuroxime (see Cefuroxime) or Cefpodoxime (see Cefpodoxime) and Macrolide (see Macrolides) or Doxycycline (see Doxycycline)
- Monotherapy with Respiratory Fluoroquinolone (Strong Recommendation, Moderate Quality of Evidence)
- Inpatient Adult with Non-Severe Community-Acquired Pneumonia and Prior Respiratory Isolation of Methicillin-Resistant Staphylococcus Aureus (MRSA)
- Monotherapy with Respiratory Fluoroquinolone
- Levofloxacin (see Levofloxacin): 750 mg PO/IV qday
- Moxifloxacin (see Moxifloxacin): 400 mg PO/IV qday
- Combination Therapy with β-Lactam and Macrolide (or Doxycycline) (Strong Recommendation, High Quality of Evidence for Combination Therapy with Macrolide; Conditional Recommendation, Low Quality of Evidence for Combination Therapy with Doxycycline)
- Ampicillin-Sulbactam (see Ampicillin-Sulbactam) or Cefotaxime (see Cefotaxime) or Ceftaroline (see Ceftaroline) or Ceftriaxone (see Ceftriaxone) and Macrolide (see Macrolide) or Doxycycline (see Doxycycline)
- Ampicillin-Sulbactam (see Ampicillin-Sulbactam: 1.5–3 g IV q6hrs
- Cefotaxime (see Cefotaxime): 1–2 g IV q8hrs
- Ceftaroline (see Ceftaroline): 600 mg q12hrs
- Ceftriaxone (see Ceftriaxone): 1–2 g IV qday
- Azithromycin (see Azithromycin): 500 mg on first day, then 250 mg PO qday (or 500 mg IV qday x ≥5 days)
- Clarithromycin (see Clarithromycin): 500 mg PO BID (or extended-release 1,000 mg PO qday)
- Doxycycline (see Doxycycline): 100 mg PO/IV BID
- Ampicillin-Sulbactam (see Ampicillin-Sulbactam) or Cefotaxime (see Cefotaxime) or Ceftaroline (see Ceftaroline) or Ceftriaxone (see Ceftriaxone) and Macrolide (see Macrolide) or Doxycycline (see Doxycycline)
- Add Methicillin-Resistant Staphylococcus Aureus Coverage and Obtain Cultures/Nasal PCR to Allow Deescalation or Confirm Need for Continued Methicillin-Resistant Staphylococcus Aureus (MRSA) Therapy
- Linezolid (see Linezolid): 600 mg PO/IV q12hrs
- Vancomycin (see Vancomycin): 15 mg/kg IV q12hrs (adjust based on levels)
- Monotherapy with Respiratory Fluoroquinolone
- Inpatient Adult with Non-Severe Community-Acquired Pneumonia and Recent Hospitalization with Intravenous Antibiotics and Locally Validated Risk Factors for Methicillin-Resistant Staphylococcus Aureus (i.e Whether Methicillin-Resistant Staphylococcus Aureus is Prevalent in Patients with Community-Acquired Pneumonia and What Risk Factors for Infection are at a Local Hospital/Catchment Area Level) (Strong Recommendation, Moderate Quality of Evidence)
- Monotherapy with Respiratory Fluoroquinolone
- Levofloxacin (see Levofloxacin): 750 mg PO/IV qday
- Moxifloxacin (see Moxifloxacin): 400 mg PO/IV qday
- Combination Therapy with β-Lactam and Macrolide (or Doxycycline)
- Ampicillin-Sulbactam (see Ampicillin-Sulbactam) or Cefotaxime (see Cefotaxime) or Ceftaroline (see Ceftaroline) or Ceftriaxone (see Ceftriaxone) and Macrolide (see Macrolide) or Doxycycline (see Doxycycline)
- Ampicillin-Sulbactam (see Ampicillin-Sulbactam: 1.5–3 g IV q6hrs
- Cefotaxime (see Cefotaxime): 1–2 g IV q8hrs
- Ceftaroline (see Ceftaroline): 600 mg q12hrs
- Ceftriaxone (see Ceftriaxone): 1–2 g IV qday
- Azithromycin (see Azithromycin): 500 mg on first day, then 250 mg PO qday (or 500 mg IV qday x ≥5 days)
- Clarithromycin (see Clarithromycin): 500 mg PO BID (or extended-release 1,000 mg PO qday)
- Doxycycline (see Doxycycline): 100 mg PO/IV BID
- Ampicillin-Sulbactam (see Ampicillin-Sulbactam) or Cefotaxime (see Cefotaxime) or Ceftaroline (see Ceftaroline) or Ceftriaxone (see Ceftriaxone) and Macrolide (see Macrolide) or Doxycycline (see Doxycycline)
- Obtain Cultures, But Withhold Methicillin-Resistant Staphylococcus Aureus Coverage Unless Culture Results are Positive
- If Methicillin-Resistant Staphylococcus Aureus Rapid Nasal PCR Testing is Negative, Withhold Additional Empiric Therapy Against Methicillin-Resistant Staphylococcus Aureus
- If Methicillin-Resistant Staphylococcus Aureus Rapid Nasal PCR Testing is Positive, Add Methicillin-Resistant Staphylococcus Aureus Coverage and Obtain Cultures
- Monotherapy with Respiratory Fluoroquinolone
- Inpatient Adult with Severe Community-Acquired Pneumonia and with Prior Respiratory Isolation of Methicillin-Resistant Staphylococcus Aureus (MRSA) or Recent Hospitalization with Intravenous Antibiotics and Locally Validated Risk Factors for Methicillin-Resistant Staphylococcus Aureus (MRSA) Therapy (i.e. Whether Methicillin-Resistant Staphylococcus Aureus is Prevalent in Patients with Community-Acquired Pneumonia and What Risk Factors for Infection are Present at a Local Hospital/Catchment Area Level) (Strong Recommendation, Moderate Quality of Evidence)
- Combination Therapy with β-Lactam and Macrolide (or Respiratory Fluoroquinolone)
- Ampicillin-Sulbactam (see Ampicillin-Sulbactam) or Cefotaxime (see Cefotaxime) or Ceftaroline (see Ceftaroline) or Ceftriaxone (see Ceftriaxone) and Macrolide (see Macrolide) or Respiratory Fluoroquinolone (see Fluoroquinolones)
- Add Methicillin-Resistant Staphylococcus Aureus (MRSA) Coverage and Obtain Cultures/Nasal PCR to Allow Deescalation or Confirmation of Need for Continued Methicillin-Resistant Staphylococcus Aureus MRSA) Therapy
- Linezolid (see Linezolid): 600 mg PO/IV q12hrs
- Vancomycin (see Vancomycin): 15 mg/kg IV q12hrs (adjust based on levels)
- Combination Therapy with β-Lactam and Macrolide (or Respiratory Fluoroquinolone)
Methicillin-Resistant Staphylococcus Aureus (MRSA) Empyema (Pleural Effusion-Parapneumonic)
Recommendations (Infectious Diseases Society of America 2011 Guidelines for the Treatment of MRSA) (Clin Infect Dis. 2011) [MEDLINE]
- Recommended Agents: as above for MRSA pneumonia
- Pleural Drainage (A-III Recommendation)
- Chest Tube (see Chest Tube)
- Surgical Pleural Drainage/Decortication
Methicillin-Resistant Staphylococcus Aureus (MRSA) Meningitis (see Meningitis)
Recommendations (Infectious Diseases Society of America 2011 Guidelines for the Treatment of MRSA) (Clin Infect Dis, 2011) [MEDLINE]
- Recommended Agents
- Vancomycin IV (see Vancomycin): 2 wk course (B-II Recommendation)
- Linezolid (Zyvox) IV/PO (see Linezolid): 600 mg BID (B-II Recommendation)
- Sulfamethoxazole-Trimethoprim (Bactrim, Septra) IV (see Sulfamethoxazole-Trimethoprim): 5 mg/kg q8-12 hrs (C-III Recommendation)
- Other Recommendations
- Optional Addition of Rifampin PO/IV (see Rifampin): 600 mg BID (B-III Recommendation)
- Recommended by Some Experts
- For Central Nervous System Shunt Infection: shunt removal is recommended and it should not be replaced until cerebrospinal fluid cultures are repeatedly negative (A-II Recommendation)
- Optional Addition of Rifampin PO/IV (see Rifampin): 600 mg BID (B-III Recommendation)
Methicillin-Resistant Staphylococcus Aureus (MRSA) Brain Abscess/Intracranial Epidural Abscess/Subdural Empyema/Spinal Epidural Abscess (see Brain Abscess, Intracranial Epidural Abscess, Subdural Empyema, and Spinal Epidural Abscess)
Recommendations (Infectious Diseases Society of America 2011 Guidelines for the Treatment of MRSA) (Clin Infect Dis, 2011) [MEDLINE]
- Recommended Agents
- Vancomycin IV (see Vancomycin): 4-6 wk course (B-II Recommendation)
- Linezolid (Zyvox) IV/PO (see Linezolid): 600 mg BID (B-II Recommendation)
- Sulfamethoxazole-Trimethoprim (Bactrim, Septra) IV (see Sulfamethoxazole-Trimethoprim): 5 mg/kg q8-12 hrs (C-III Recommendation)
- Other Recommendations
- Optional Addition of Rifampin PO/IV (see Rifampin): 600 mg qday or 300-450 mg BID (B-III Recommendation)
- Recommended by Some Experts
- Neurosurgical Evaluation for Incision and Drainage is Recommended (A-II Recommendation)
- Optional Addition of Rifampin PO/IV (see Rifampin): 600 mg qday or 300-450 mg BID (B-III Recommendation)
Methicillin-Resistant Staphylococcus Aureus (MRSA) Septic Thrombosis of Cavernous or Dural Venous Sinus (see xxxx)
Recommendations (Infectious Diseases Society of America 2011 Guidelines for the Treatment of MRSA) (Clin Infect Dis, 2011) [MEDLINE]
- Recommended Agents
- Vancomycin IV (see Vancomycin): 4-6 wk course (B-II Recommendation)
- Linezolid (Zyvox) IV/PO (see Linezolid): 600 mg BID (B-II Recommendation)
- Sulfamethoxazole-Trimethoprim (Bactrim, Septra) IV (see Sulfamethoxazole-Trimethoprim): 5 mg/kg q8-12 hrs (C-III Recommendation)
- Other Recommendations
- Optional Addition of Rifampin PO/IV (see Rifampin): 600 mg qday or 300-450 mg BID (B-III Recommendation)
- Recommended by Some Experts
- Neurosurgical Evaluation for Incision and Drainage of Contiguous Sites is Recommended (A-II Recommendation)
- Role of Anticoagulation is Controversial
- Optional Addition of Rifampin PO/IV (see Rifampin): 600 mg qday or 300-450 mg BID (B-III Recommendation)
Methicillin-Resistant Staphylococcus Aureus (MRSA) Osteomyelitis (see Osteomyelitis)
Recommendations (Infectious Diseases Society of America 2011 Guidelines for the Treatment of MRSA) (Clin Infect Dis, 2011) [MEDLINE]
- Agents
- Vancomycin IV (see Vancomycin) (B-II Recommendation)
- Daptomycin (Cubicin) (see Daptomycin): 6 mg/kg/dose IV qday at least 8 wk course (B-II Recommendation)
- Linezolid (Zyvox) IV/PO (see Linezolid): 600 mg BID (B-II Recommendation)
- Clindamycin IV/PO (see Clindamycin): 600 mg TID (B-III Recommendation)
- Combination Sulfamethoxazole-Trimethoprim (Bactrim, Septra) 4 mg/kg/dose PO BID + Rifampin 600 mg PO qday (see Sulfamethoxazole-Trimethoprim and Rifampin) (B-II Recommendation)
- Other Recommendations
- Surgical Debridement and Drainage of Associated Soft Tissue Abscesses is Recommended (A-II Recommendation)
- Optional Addition of Rifampin PO/IV (see Rifampin): 600 mg qday or 300-450 mg BID (B-III Recommendation)
- Recommended by Some Experts
- For Patients with Concurrent Bacteremia, Rifampin Should Be Added After the Clearance of the Bacteremia
- Optimal Route of Antibiotic Administration is Unknown (A-III Recommendation)
- Optimal Duration of Antibiotic Administration is Unknown: at least 8 wk course is recommended (A-II recommendation)
- Some Experts Recommend an Additional 1-3 mo (and Possibly Longer for Chronic Infection or if Debridement is Not Performed) of Oral Rifampin-Based Combination Therapy with Sulfamethoxazole-Trimethoprim, Doxycycline/Minocycline, Clindamycin, or Fluoroquinolone Chosen Based on Susceptibilities (C-III Recommendation)
- Magnetic Resonance Imaging is the Imaging Procedure of Choice, Particularly for the Detection of Early Osteomyelitis and/or Associated Soft Tissue Disease (A-II Recommendation)
- Erythrocyte Sedimentation Rate (ESR) and C-Reactive Protein (CRP) May Be Helpful to Guide Response to Therapy (B-III Recommendation)
Methicillin-Resistant Staphylococcus Aureus (MRSA) Septic Arthritis (see Septic Arthritis)
Recommendations (Infectious Diseases Society of America 2011 Guidelines for the Treatment of MRSA) (Clin Infect Dis, 2011) [MEDLINE]
- Agents: same as for osteomyelitis above
- Other Recommendations
- Debridement and Drainage of Joint Space is Recommended in All Cases (A-II Recommendation)
- Duration of Antibiotic Therapy: 3-4 wk course is suggested (A-III Recommendation)
Methicillin-Resistant Staphylococcus Aureus (MRSA) Device-Related Osteoarticular Infection
Recommendations (Infectious Diseases Society of America 2011 Guidelines for the Treatment of MRSA) (Clin Infect Dis, 2011) [MEDLINE]
- Early-Onset (<2 mo Post-Op) or Acute Hematogenous Prosthetic Joint Infections with a Stable Implant of Short Duration (≤3 wks) of Symptoms and Debridement (But Device Retention)
- Agents: same as for osteomyelitis above with the addition of Rifampin 600 mg PO qday or 300-450 mg PO BID x 2 wks, followed by Rifampin + Fluoroquinolone/Sulfamethoxazole-Trimethoprim/Tetracycline/Clindamycin x 3 mo (for hip) or 6 mo (for knee) (A-II Recommendation)
- Late-Onset Infection, Unstable Implant, or Long Duration of Symptoms with Hip/Knee Implant: device removal is recommended (A-II Recommendation)
- Early-Onset (≤30 Days Post-Op) Spinal Implant Infection or Implant in Actively Infected Site: same as for osteomyelitis above with the addition of Rifampin, followed by prolonged oral therapy (B-II Recommendation)
- Optimal Duration of Antibiotic Administration is Unknown: oral component should be continued until spine fusion has occurred (B-II Recommendation)
- Late-Onset (>30 Days Post-Op) Spinal Implant Infection: device removal is recommended (B-II Recommendation)
- Long-Term Oral Suppressive Therapy: Sulfamethoxazole-Trimethoprim/Tetracycline/Fluoroquinolone/Clindamycin in combination with Rifampin are recommended (particularly if device removal is not possible
Other Therapies
Recommendations (Infectious Diseases Society of America 2011 Guidelines for the Treatment of MRSA) (Clin Infect Dis, 2011) [MEDLINE]
- Intravenous Immunoglobulin (IVIG) (see Intravenous Immunoglobulin): not routinely recommended for invasive MRSA disease (A-III Recommendation), although may be used as adjunctive therapy in selected clinical scenarios, such as necrotizing fasciitis or severe sepsis (C-III Recommendation)
- Protein Synthesis Inhbitors: not routinely recommended for invasive MRSA disease (A-III Recommendation), although may be used as adjunctive therapy in selected clinical scenarios, such as necrotizing fasciitis or severe sepsis (C-III Recommendation)
- Clindamycin (see Clindamycin)
- Linezolid (Zyvox) (see Linezolid)
- Tedizolid (Sivextro) (see Tedizolid)
Specific Agents with Activity Against Methicillin-Resistant Staphylococcus Aureus (MRSA)

Vancomycin (see Vancomycin)
- Tissue Penetration is Highly Variable and Dependent on the Degree of Inflammation
- Limited Penetration into Bone
- Limited Penetration into Lung Epithelial Fluid
- Limited Penetration into Cerebrospinal Fluid
- Vancomycin Administration Recommendations (Infectious Diseases Society of America 2011 Guidelines for the Treatment of MRSA) (Clin Infect Dis, 2011) [MEDLINE]
- Standard Vancomycin Dosing: 15-20 mg/kg/dose (Actual Body Weight) q8-12 hrs (Max: 2 g Per Dose) in Patients with Normal Renal Function (B-III Recommendation)
- Dosing in Patients with Serious Disease (Sepsis, Meningitis, Pneumonia, Infective Endocarditis): loading dose of 25-30 mg/kg (actual body weight) may be considered
- Due to Risk of Red Man Syndrome and Possible Anaphylaxis with Large Vancomycin Dose, Prophylactic Antihistamine Should Be Used and Vancomycin Infusion Time Should Be Prolonged to 2 hrs
- Dosing in Patients with Serious Disease (Sepsis, Meningitis, Pneumonia, Infective Endocarditis): loading dose of 25-30 mg/kg (actual body weight) may be considered
- Monitor Serum Vancomycin Trough Levels: troughs are the most accurate method to guide vancomycin dosing (B-II Recommendation)
- Vancomycin Trough Should Be Obtained at Steady State Condition Prior to 4th-5th Dose
- Target Vancomycin Trough Level in Serious Infections: 15-20 μg/mL (B-II Recommendation)
- Patients with Skin and Soft Tissue Infections (SSTI) and Normal Renal Function: standard dosing of 1g q12hrs is adequate and trough level measurement is not necessary (B-II Recommendation)
- Vancomycin Trough Measurement is Recommended in Patients with Serious Infections, Who are Morbidly Obese, Have Renal Insufficiency (Including Those on Dialysis), and Those with Fluctuating Volumes of Distribution (A-II Recommendation)
- Measurement of Peak Serum Vancomycin Levels are Not Recommended (B-II Recommendation)
- Continuous Vancomycin Infusion Regimens are Not Recommended (A-II Recommendation)
- Standard Vancomycin Dosing: 15-20 mg/kg/dose (Actual Body Weight) q8-12 hrs (Max: 2 g Per Dose) in Patients with Normal Renal Function (B-III Recommendation)
- Vancomycin Susceptibility Testing (Infectious Diseases Society of America 2011 Guidelines for the Treatment of MRSA) (Clin Infect Dis, 2011) [MEDLINE]
- Isolates with Vancomycin Minimum Inhibitory Concentration (MIC) ≤2 μg/mL (Susceptible): clinical response should determine the continued use of vancomycin (A-III Recommendation)
- If Patient Does Not Have Clinical/Microbiologic Response Despite Adequate Debridement and Removal of Other Foci of Infection, an Alternative to Vancomycin is Recommended, Regardless of the MIC
- Isolates with Vancomycin Minimum Inhibitory Concentration (MIC) >2 μg/mL (Vancomycin-Intermediate Staphylococcus Aureus, VISA, or Vancomycin-Resistant Staphylococcus Aureus, VRSA): an alternative to vancomycin should be used (A-III Recommendation)
- Isolates with Vancomycin Minimum Inhibitory Concentration (MIC) ≤2 μg/mL (Susceptible): clinical response should determine the continued use of vancomycin (A-III Recommendation)
Linezolid (see Linezolid)
- Clinical Efficacy
- Retrospective Analysis Comparing Linezolid with Vancomycin for MRSA Hospital-Acquired Pneumonia (Chest, 2003) [MEDLINE]
- Initial Therapy with Linezolid was Associated with Improved Survival and Cure Rates, as Compared to Vancomycin
- Comparison of Linezolid with Vancomycin in the Treatment of MRSA Ventilator-Associated Pneumonia (Chest, 2008) [MEDLINE]
- No Difference in Early Microbiological Cure Rates with Linezolid, as Compared to Vancomycin
- Trends in the Secondary Outcomes Appeared to Favor Linezolid
- No Difference in Early Microbiological Cure Rates with Linezolid, as Compared to Vancomycin
- Retrospective Analysis Comparing Linezolid with Vancomycin for MRSA Hospital-Acquired Pneumonia (Chest, 2003) [MEDLINE]
Tedizolid (Sivextro) (see Tedizolid)
- XXXXXXXX
Tigecycline (see Tigecycline)
- FDA-Approved for the Treatment of cSSTIs and Intraabdominal Infections
- Clinical Efficacy
Dalbavancin (Dalvance) (see Dalbavancin)
- XXXXX
Daptomycin (see Daptomycin)
- Clinical Features
- FDA-approved to treat skin and soft tissue infections due to Gram-positive organisms
- FDA-approved to treat endocarditis due to Staphyloccocus Aureus
Ceftaroline (Teflaro, Zinfloro) (see Ceftaroline)
- Pharmacology
- XXX
- Penetration
- XXX
- Side Effects
- XXX
- Contraindications
- XXX
Telavancin (Vibativ) (see Telavancin)
- Pharmacology
- XXX
- Penetration
- XXX
- Clinical Features
- XXX
- Side Effects
- XXX
- Contraindications
- XXX
Prognosis
Methicillin-Sensitive Staphylococcus Aureus Infection (MSSA) vs Methicillin-Resistant Staphylococcus Aureus (MRSA)
- Single VA Study of the Outcomes of Staphylococcus Aureus Infection (Infect Control Hosp Epidemiol, 2007) [MEDLINE]: n = 438
- Patients with Non-Pneumonia MRSA Infections Have a Higher Mortality Rate than Patients with Non-Pneumonia MSSA Infections, Independent of the Severity of Patient’s Underlying Illness
- Mortality Rate MRSA with Panton-Valentine Leukocidin Toxin: 75%
- Mortality Rate with Associated Bacteremia: 30%
References
General
- Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019 Oct 1;200(7):e45-e67. doi: 10.1164/rccm.201908-1581ST [MEDLINE]
Epidemiology
- Methicillin-resistant Staphylococcus aureus: a consensus review of the microbiology, pathogenesis, and epidemiology with implications for prevention and management. Am J Med 1993; 94:313-328
- Fifteen-year study of the changing epidemiology of methicillin-resistant Staphylococcus aureus. Am J Med 2006; 119:943
Physiology
- Methicillin-resistant staphylococci. J Clin Pathol. 1961;14:385 [MEDLINE]
- Prospective study of infection, colonization and carriage of methicillin-resistant Staphylococcus aureus in an outbreak affecting 990 patients. Eur J Clin Microbiol Infect Dis. 1994;13(1):74 [MEDLINE]
- Nosocomial Staphylococcus aureus bacteremia among nasal carriers of methicillin-resistant and methicillin-sensitive strains. Am J Med 1996; 100:509-516
- Improved methods for detection of methicillin-resistant Staphylococcus aureus. Eur J Clin Microbiol Infect Dis. 2001;20(4):267 [MEDLINE]
- National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;32(8):470 [MEDLINE]
- Methicillin-resistant Staphylococcus aureus (MRSA) nares colonization at hospital admission and its effect on subsequent MRSA infection. Clin Infect Dis. 2004;39(6):776 [MEDLINE]
- Duration of methicillin-resistant Staphylococcus aureus carriage, according to risk factors for acquisition. Infect Control Hosp Epidemiol. 2006 Nov;27(11):1206-12 [MEDLINE]
- Concurrent analysis of nose and groin swab specimens by the IDI-MRSA PCR assay is comparable to analysis by individual-specimen PCR and routine culture assays for detection of colonization by methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 2006;44(8):2904 [MEDLINE]
- Evaluation of a new chromogenic medium for the detection of methicillin-resistant Staphylococcus aureus carriage on nasal and perianal specimens. Diagn Microbiol Infect Dis. 2008;60(2):225 [MEDLINE]
- Changes in the prevalence of nasal colonization with Staphylococcus aureus in the United States, 2001-2004. J Infect Dis. 2008;197(9):1226 [MEDLINE]
- A prevalence study of methicillin-resistant Staphylococcus aureus colonization in emergency department health care workers. Ann Emerg Med. 2008;52(5):52 [MEDLINE]
- Prevalence of Staphylococcus aureus nasal colonization in emergency department personnel. Ann Emerg Med. 2008;52(5):529 [MEDLINE]
- Carriage of methicillin-resistant Staphylococcus aureus in home care settings: prevalence, duration, and transmission to household members. Arch Intern Med. 2009;169(15):1372 [MEDLINE]
- Exclusive Staphylococcus aureus throat carriage: at-risk populations. Arch Intern Med. 2009;169(2):172 [MEDLINE]
- Duration of colonization with methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2009;48(7):910 [MEDLINE]
- National prevalence of methicillin-resistant Staphylococcus aureus in inpatients at United States health care facilities, 2010. Am J Infect Control. 2012 Apr;40(3):194-200 [MEDLINE]
- Natural history of colonization with methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE): a systematic review. BMC Infect Dis. 2014;14:177 [MEDLINE]
- Duration of colonization with methicillin-resistant Staphylococcus aureus in an acute care facility: a study to assess epidemiologic features. Am J Infect Control. 2014 Mar;42(3):249-53 [MEDLINE]
- Duration of Colonization and Determinants of Earlier Clearance of Colonization With Methicillin-Resistant Staphylococcus aureus. Clin Infect Dis. 2015 May;60(10):1489-96 [MEDLINE]
- Cessation from Smoking Improves Innate Host Defense and Clearance of Experimentally Inoculated Nasal Staphylococcus aureus. Infect Immun. 2018 Mar 22;86(4):e00912-17. doi: 10.1128/IAI.00912-17. Print 2018 Apr [MEDLINE]
- Effect of nicotine on Staphylococcus aureus biofilm formation and virulence factors. Sci Rep. 2019 Dec 27;9(1):20243. doi: 10.1038/s41598-019-56627-0 [MEDLINE]
- Methicillin-resistant Staphylococcus aureus Colonization and Pre- and Post-hospital Discharge Infection Risk. Clin Infect Dis. 2019;68(4):545 [MEDLINE]
Microbiology
- Novel type of staphylococcal cassette chromosome mec identified in community-acquired methicillin-resistant Staphylococcus aureus strains. Antimicrob Agents Chemother. 2002;46(4):1147 [MEDLINE]
- Genome and virulence determinants of high virulence community-acquired MRSA. Lancet. 2002;359(9320):1819 [MEDLINE]
- Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352(14):1436 [MEDLINE]
- Roles of 34 virulence genes in the evolution of hospital- and community-associated strains of methicillin-resistant Staphylococcus aureus. J Infect Dis. 2006;193(11):1495 [MEDLINE]
- Characterization of a strain of community-associated methicillin-resistant Staphylococcus aureus widely disseminated in the United States. J Clin Microbiol. 2006;44(1):108 [MEDLINE]
- Emergence of community-acquired methicillin-resistant Staphylococcus aureus USA 300 clone as the predominant cause of skin and soft-tissue infections. Ann Intern Med. 2006;144(5):309 [MEDLINE]
- Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet. 2006;367(9512):731 [MEDLINE]
- Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298(15):1763 [MEDLINE]
- Emergence of multidrug-resistant, community-associated, methicillin-resistant Staphylococcus aureus clone USA300 in men who have sex with men. Ann Intern Med. 2008;148(4):249 [MEDLINE]
Diagnosis
- The Clinical Utility of Methicillin-Resistant Staphylococcus aureus (MRSA) Nasal Screening to Rule Out MRSA Pneumonia: A Diagnostic Meta-analysis With Antimicrobial Stewardship Implications. Clin Infect Dis . 2018 Jun 18;67(1):1-7. doi: 10.1093/cid/ciy024 [MEDLINE]
Clinical Manifestations
Cardiovascular Manifestations
- Prosthetic valve endocarditis resulting from nosocomial bacteremia. A prospective, multicenter study. Ann Intern Med. 1993;119(7 Pt 1):560 [MEDLINE]
- Role of echocardiography in evaluation of patients with Staphylococcus aureus bacteremia: experience in 103 patients. J Am Coll Cardiol. 1997;30(4):1072 [MEDLINE]
- Prospective analysis of Staphylococcus aureus bacteremia in nonneutropenic adults with malignancy. J Clin Oncol. 2000;18(5):1110 [MEDLINE]
- Prospective analysis of Staphylococcus aureus bacteremia in nonneutropenic adults with malignancy. J Clin Oncol. 2000;18(5):1110 [MEDLINE]
- Clinical identifiers of complicated Staphylococcus aureus bacteremia. Arch Intern Med. 2003;163(17):2066 [MEDLINE]
- A prospective multicenter study of Staphylococcus aureus bacteremia: incidence of endocarditis, risk factors for mortality, and clinical impact of methicillin resistance. Medicine (Baltimore). 2003;82(5):322 [MEDLINE]
- Staphylococcus aureus bacteremia and endocarditis: the Grady Memorial Hospital experience with methicillin-sensitive S aureus and methicillin-resistant S aureus bacteremia. Am Heart J. 2004;147(3):536 [MEDLINE]
- Importance of transesophageal echocardiography in the evaluation of Staphylococcus aureus bacteremia. J Heart Valve Dis. 2005;14(1):23 [MEDLINE]
- Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA. 2005;293(24):3012 [MEDLINE]
- Comparison of clinical and morphological characteristics of Staphylococcus aureus endocarditis with endocarditis caused by other pathogens. Heart. 2005;91(7):932 [MEDLINE]
- Risk of endocarditis among patients with prosthetic valves and Staphylococcus aureus bacteremia. Am J Med. 2005;118(3):225 [MEDLINE]
- Risk factors for infective endocarditis and outcome of patients with Staphylococcus aureus bacteremia. Mayo Clin Proc. 2007;82(10):1165 [MEDLINE]
- Clinical management of Staphylococcus aureus bacteremia: a review. JAMA. 2014 Oct;312(13):1330-41 [MEDLINE]
- Staphylococcus aureus Bloodstream Infection and Endocarditis–A Prospective Cohort Study. PLoS One. 2015;10(5):e0127385 [MEDLINE]
- The causative agents in infective endocarditis: a systematic review comprising 33,214 cases. Eur J Clin Microbiol Infect Dis. 2016;35(8):1227 [MEDLINE]
- Temporal Trends of Infective Endocarditis in Olmsted County, Minnesota, Between 1970 and 2018: A Population-Based Analysis. Open Forum Infect Dis. 2021;8(3):ofab038 [MEDLINE]
Dermatologic Manifestations
- Purpura fulminans due to Staphylococcus aureus. Clin Infect Dis. 2005 Apr 1;40(7):941-7 [MEDLINE]
- Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. 2015 Jul;28(3):603-61 [MEDLINE]
- Methicillin-resistant Staphylococcus aureus: an overview of basic and clinical research. Nat Rev Microbiol. 2019;17(4):203 [MEDLINE]
Pulmonary Manifestations
Bronchitis Obliterans (see xxxx)
- Bronchitis obliterans due to Influenza B pneumonia complicated with Staphylococcus aureus infection. Arch Bronconeumol. 2017 Aug;53(8):463-464. doi: 10.1016/j.arbres.2017.01.006 [MEDLINE]
Pneumonia (see Community-Acquired Pneumonia)
- Prospective study of 114 consecutive episodes of Staphylococcus aureus bacteremia. Rev Infect Dis. 1987;9(5):891 [MEDLINE]
- Community-acquired methicillin-resistant Staphylococcus aureus infections in France: emergence of a single clone that produces Panton-Valentine leukocidin. Clin Infect Dis. 2002;35(7):819 [MEDLINE]
- Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet. 2002;359(9308):753 [MEDLINE]
- Severe community-onset pneumonia in healthy adults caused by methicillin-resistant Staphylococcus aureus carrying the Panton-Valentine leukocidin genes. Clin Infect Dis. 2005;40(1):100 [MEDLINE]
- Severe community-acquired pneumonia due to Staphylococcus aureus, 2003-04 influenza season. Emerg Infect Dis. 2006;12(6):894 [MEDLINE]
- Severe methicillin-resistant Staphylococcus aureus community-acquired pneumonia associated with influenza–Louisiana and Georgia, December 2006-January 2007. MMWR Morb Mortal Wkly Rep. 2007;56(14):325 [MEDLINE]
- Comparison of mortality risk associated with bacteremia due to methicillin-resistant and methicillin-susceptible Staphylococcus aureus. Infect Control Hosp Epidemiol. 2007;28(3):273 [MEDLINE]
- Factors predicting mortality in necrotizing community-acquired pneumonia caused by Staphylococcus aureus containing Panton-Valentine leukocidin. Clin Infect Dis. 2007;45(3):315 [MEDLINE]
- Pneumonia caused by methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2008;46 Suppl 5:S378 [MEDLINE]
- Contribution of Panton-Valentine leukocidin in community-associated methicillin-resistant Staphylococcus aureus pathogenesis. PLoS One. 2008;3(9):e3198 [MEDLINE]
- Staphylococcus aureus community-acquired pneumonia during the 2006 to 2007 influenza season. Ann Emerg Med. 2009;53(3):358 [MEDLINE]
- Comparative analysis of virulence and toxin expression of global community-associated methicillin-resistant Staphylococcus aureus strains. J Infect Dis. 2010;202(12):1866 [MEDLINE]
- Community-associated meticillin-resistant Staphylococcus aureus. Lancet. 2010;375(9725):1557 [MEDLINE]
- Lack of a major role of Staphylococcus aureus Panton-Valentine leukocidin in lower respiratory tract infection in nonhuman primates. Am J Pathol. 2010;176(3):1346 [MEDLINE]
- Expanded clinical presentation of community-acquired methicillin-resistant Staphylococcus aureus pneumonia. Chest. 2010;138(1):130 [MEDLINE]
- Severity of disease and clinical outcomes in patients with hospital-acquired pneumonia due to methicillin-resistant Staphylococcus aureus strains not influenced by the presence of the Panton-Valentine leukocidin gene. Clin Infect Dis. 2011;53(8):766 [MEDLINE]
- EMERGEncy ID NET Study. Prevalence of methicillin-resistant staphylococcus aureus as an etiology of community-acquired pneumonia. Clin Infect Dis. 2012;54(8):1126 [MEDLINE]
- How important is methicillin-resistant Staphylococcus aureus as a cause of community-acquired pneumonia and what is best antimicrobial therapy? Infect Dis Clin North Am. 2013 Mar;27(1):177-88 [MEDLINE]
- Global initiative for meticillin-resistant Staphylococcus aureus pneumonia (GLIMP): an international, observational cohort study. Lancet Infect Dis. 2016 Dec;16(12):1364-1376 [MEDLINE]
- Staphylococcus aureus Community-acquired Pneumonia: Prevalence, Clinical Characteristics, and Outcomes. Clin Infect Dis. 2016;63(3):300 [MEDLINE]
Tracheobronchitis (see Acute Bronchitis)
- Treatment of acute bacterial exacerbations of chronic obstructive pulmonary disease in hospitalised patients–a comparison of meropenem and imipenem/cilastatin. COPD Study Group. J Antimicrob Chemother. 1995 Jul;36 Suppl A:121-33. doi: 10.1093/jac/36.suppl_a.121 [MEDLINE]
- Fatal influenza A infection with Staphylococcus aureus superinfection in a 49-year-old woman presenting as sudden death. Int J Legal Med. 2005 Jan;119(1):40-3. doi: 10.1007/s00414-004-0472-1 [MEDLINE]
- Study entry microbiology in patients with acute bacterial exacerbation of chronic bronchitis in a clinical trial stratifying by disease severity. Curr Med Res Opin. 2007 Jan;23(1):1-7. doi: 10.1185/030079907X159515 [MEDLINE]
- Tracheobronchitis caused by methicillin-resistant Staphylococcus aureus as a cause of chronic wheezing in a non-ventilated adult patient with tracheobronchomalacia. Respiration. 2010;80(2):148-56. doi: 10.1159/000273268 [MEDLINE]
- Purulent Bronchitis Caused by Staphylococcus aureus in an Adolescent Receiving Infliximab for Crohn’s Disease. Klin Padiatr. 2015 Jul;227(4):232-3. doi: 10.1055/s-0035-1548748 [MEDLINE]
- A Case of Severe Pseudomembranous Tracheobronchitis Complicated by Co-infection of Influenza A (H1N1) and Staphylococcus aureus in an Immunocompetent Patient. Tuberc Respir Dis (Seoul). 2015 Oct;78(4):366-70. doi: 10.4046/trd.2015.78.4.366 [MEDLINE]
- Severe Necrotizing Tracheobronchitis From Panton-Valentine Leukocidin-positive MRSA Pneumonia Complicating Influenza A-H1N1-09. J Bronchology Interv Pulmonol. 2017 Jan;24(1):63-66. doi: 10.1097/LBR.0000000000000314 [MEDLINE]
- Necrotizing tracheobronchitis caused by influenza and Staphylococcus aureus co-infection. Infection. 2018 Oct;46(5):737-739. doi: 10.1007/s15010-018-1180-y [MEDLINE]
Vascular Manifestations
- Staphylococcus aureus infections. N Engl J Med. 1998;339(8):520 [MEDLINE]
- Risk factors for hematogenous complications of intravascular catheter-associated Staphylococcus aureus bacteremia. Clin Infect Dis. 2005;40(5):695 [MEDLINE]
- Changing Characteristics of Staphylococcus aureus Bacteremia: Results From a 21-Year, Prospective, Longitudinal Study. Clin Infect Dis. 2019;69(11):1868 [MEDLINE]
Prevention
- Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med. 2011 Apr 14;364(15):1419-30. doi: 10.1056/NEJMoa1007474 [MEDLINE]
- Infection prevention practices in adult intensive care units in a large community hospital system after implementing strategies to reduce healthcare-associated methicillin-resistant Staphylococcus aureus infections. Am J Infect Control 2013;41:126-130 [MEDLINE]
- REDUCE MRSA Trial. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med. 2013;368:2255–2265 [MEDLINE]
- Screening inpatients for MRSA—case closed. N Engl J Med. 2013;368:2314–2315 [MEDLINE]
- Effect of body surface decolonisation on bacteriuria and candiduria in intensive care units: an analysis of a cluster-randomised trial. Lancet Infect Dis. 2016 Jan;16(1):70-9. doi: 10.1016/S1473-3099(15)00238-8. Epub 2015 Nov 27 [MEDLINE]
Treatment
- Linezolid versus vancomycin: analysis of two double blind studies of patients with MRSA pneumonia. Chest 2003;124:1787 [MEDLINE]
- Results of a multicenter, randomized, open-label efficacy and safety study of two doses of tigecycline for complicated skin and skin-structure infections in hospitalized patients. Clin Ther. 2004 May;26(5):704-14 [MEDLINE]
- Antimicrobial activity of tigecycline tested against organisms causing community-acquired respiratory tract infection and nosocomial pneumonia. Diagn Microbiol Infect Dis. 2005 Jul;52(3):187-93 [MEDLINE]
- Epidemiology, treatment, and outcomes of nosocomial bacteremic Staphylococcus aureus pneumonia. Chest. 2005 Sep;128(3):1414-22 [MEDLINE]
- Linezolid versus vancomycin in treatment of complicated skin and soft tissue infections. Antimicrob Agents Chemother. 2005; 49:2260-2266 [MEDLINE]
- High-dose vancomycin therapy for MRSA infections: efficacy and toxicity. Arch Intern Med 2006;123:2138-2144 [MEDLINE]
- In vitro activities of ceftobiprole, tigecycline, daptomycin, and 19 other antimicrobials against methicillin-resistant Staphylococcus aureus strains from a national survey of Belgian hospitals. Antimicrob Agents Chemother. 2006 Aug;50(8):2680-5 [MEDLINE]
- Early Microbiologic Response to Linezolid Versus Vancomycin in Ventilator-Associated Pneumonia (VAP) Due to Methicillin-Resistant Staphylococcus aureus (MRSA) Chest 2008 Dec;134(6):1200-7 [MEDLINE]
- Impact of methicillin resistance on mortality in Staphylococcus aureus VAP: a systematic review. Eur Respir J. 2008 Mar;31(3):625-32 [MEDLINE]
- Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011 Feb 1;52(3):e18-55. doi: 10.1093/cid/ciq146. Epub 2011 Jan 4 [MEDLINE]
- US Food and Drug Administration. FDA Drug Safety Communication. Increased risk of death with Tygacil (tigecycline) compared to other antibiotics used to treat similar infections. 2010. Available at: https:// www.fda.gov/Drugs/DrugSafety/ucm224370.htm
- Systematic review and meta-analysis of the effectiveness and safety of tigecycline for treatment of infec- tious disease. Antimicrob Agents Chemother 2011; 55:1162–72
- Efficacy and safety of tigecycline for the treatment of infectious diseases: a meta-analysis. Lancet Infect Dis 2011; 11:834–44
- Efficacy and safety of tigecy- cline: a systematic review and meta-analysis. J Antimicrob Chemother 2011; 66:1963–71
- Excess deaths associated with tigecycline after approval based on noninferiority trials. Clin Infect Dis 2012; 54:1699–709 [MEDLINE]
- Methicillin-resistant Staphylococcus aureus therapy: past, present, and future. Clin Infect Dis. 2014 Jan;58 Suppl 1:S20-7. doi: 10.1093/cid/cit614 [MEDLINE]
- Clinical practice. Community-acquired pneumonia. N Engl J Med. 2014 Feb 6;370(6):543-51. doi: 10.1056/NEJMcp1214869 [MEDLINE]
- Management of Skin Abscesses in the Era of Methicillin-Resistant Staphylococcus aureus. N Engl J Med 2014; 370:1039-1047March 13, 2014DOI: 10.1056/NEJMra1212788 [MEDLINE]
- Empirical Anti-MRSA vs Standard Antibiotic Therapy and Risk of 30-Day Mortality in Patients Hospitalized for Pneumonia. JAMA Intern Med. 2020 Feb 17. doi: 10.1001/jamainternmed.2019.7495 [MEDLINE]
