Epidemiology
- xxx
Etiology
- Intraperitoneal Surgery:
- Most common etiology
- Longest duration after colonic surgery
- Lap surgery has shorter length of post-op ileus
- Extraperitoneal Surgery: retroperitoneal, etc
- [[Sepsis]]
- Drugs (eg, opioids, antacids, warfarin, amitriptyline, chlorpromazine)
- Metabolic (eg, low potassium, magnesium, or sodium levels; anemia; hyposmolality)
- Myocardial infarction
- Pneumonia
- Trauma (eg, fractured ribs, fractured spine)
- Biliary colic and renal colic
- Head injury and neurosurgical procedures
- Intra-abdominal inflammation and peritonitis
- [[Retroperitoneal Hematoma]]
Pathophysiology
- According to some hypotheses, postoperative ileus is mediated via activation of inhibitory spinal reflex arcs. Anatomically, 3 distinct reflexes are involved: ultrashort reflexes confined to the bowel wall, short reflexes involving prevertebral ganglia, and long reflexes involving the spinal cord.[3] The long reflexes are the most significant. Spinal anesthesia, abdominal sympathectomy, and nerve-cutting techniques have been demonstrated to either prevent or attenuate the development of ileus. The surgical stress response leads to systemic generation of endocrine and inflammatory mediators that also promote the development of ileus. Rat models have shown that laparotomy, eventration, and bowel compression lead to increased numbers of macrophages, monocytes, dendritic cells, T cells, natural killer cells, and mast cells, as demonstrated by immunohistochemistry.[7] Macrophages residing in the muscularis externa and mast cells are probably the key players in this inflammatory cascade.[8] Calcitonin gene–related peptide, nitric oxide, vasoactive intestinal peptide, and substance P function as inhibitory neurotransmitters in the bowel nervous system. Nitric oxide and vasoactive intestinal peptide inhibitors and substance P receptor antagonists have been demonstrated to improve gastrointestinal function
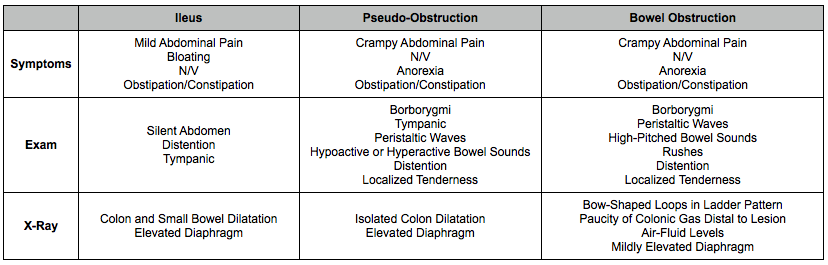
Clinical
Treatment
-
Most cases of postoperative ileus resolve with watchful waiting and supportive treatment. Patients should receive intravenous hydration. For patients with vomiting and distention, use of a nasogastric tube provides symptomatic relief; however, no studies in the literature support the use of nasogastric tubes to facilitate resolution of ileus. Long intestinal tubes have no benefit over nasogastric tubes.
-
For patients with protracted ileus, mechanical obstruction must be excluded with contrast studies. Underlying sepsis and electrolyte abnormalities, particularly hypokalemia, hyponatremia, and hypomagnesemia, may worsen ileus. These contributing conditions are easily diagnosed and corrected.
-
Discontinue medications that produce ileus (eg, opiates). In one study, the amount of morphine administered directly correlated with the time elapsed before the return of bowel sounds and the passage of flatus and stool.[16]
-
The use of postoperative narcotics can be diminished by supplementation with nonsteroidal anti-inflammatory drugs (NSAIDs). In addition to permitting lower narcotic doses by providing pain relief, NSAIDS may improve ileus by reducing local inflammation. Myoelectric activities recorded from electrodes placed on the colon have revealed faster resolution from ileus in patients given ketorolac versus those given morphine[17] ; however, the drawbacks of NSAID use include platelet dysfunction and gastric mucosal ulceration. Consider the use of a cyclooxygenase-2 selective agent (ie, celecoxib), which negates these adverse effects.
-
No single objective variable accurately predicts the resolution of ileus. The clinician must assess the overall status of the patient and evaluate for adequate oral intake and good bowel function. A patient’s report of flatus, bowel sounds, or stool passage may prove misleading; therefore, clinicians must not rely solely on self-reporting.
-
Diet: It is generally advisable to delay oral feeding until ileus resolves clinically. However, the presence of ileus does not preclude enteral feeding. Postpyloric feeding into the small bowel can be cautiously performed. Start feeds at one-quarter or one-half strength at a slow rate and gradually advance. Having patients chew gum has been advocated as a means of promoting recovery from postoperative ileus. Chewing gum may constitute a form of sham feeding that stimulates gastrointestinal motility. Meta-analyses have shown that gum chewing can reduce the time to first flatus and passage of feces, and marginally decrease the length of hospital stay after intestinal surgery
-
Activity: Conventional wisdom and wide practice foster the notion that ambulation stimulates bowel function and improves postoperative ileus, although this has not been shown in the literature. In a nonrandomized study evaluating 34 patients, seromuscular bipolar electrodes were placed in segments of the gastrointestinal tract after laparotomy. Ten patients were assigned to ambulate on postoperative day 1, and the other 24 were assigned to ambulate on postoperative day 4. No significant difference between the 2 groups was displayed in myoelectric recovery in the stomach, jejunum, or colon.[20] Hence, postoperative ambulation remains beneficial in preventing the formation of atelectasis, deep vein thrombosis, and pneumonia but has no role in treating ileus.
-
Medications: Thoracic epidural administration has been shown to be beneficial, both with open and with endoscopic colorectal surgery.[21] Epidural blockade with local anesthetics improves postoperative ileus by blockage of inhibitory reflexes and efferent sympathetics. Studies have shown that combinations of thoracic epidurals containing bupivacaine alone or in combination with opioids improve postoperative ileus.[22, 23] Continuous intravenous administration of lidocaine during and after abdominal surgery may decrease the duration of postoperative ileus.[24]
- Peripherally selective opioid antagonists are an option for the treatment of postoperative ileus.[25] Methylnaltrexone (Relistor) and alvimopan (Entereg) are approved by the Food and Drug Administration. These agents inhibit peripheral mu-opioid receptors, which abolishes the adverse gastrointestinal effects of opioids; however, because these agents do not cross the blood-brain barrier, they do not impair the analgesic effects of opioids.[26] Methylnaltrexone is indicated for opioid-induced constipation in patients with advanced illness receiving palliative care, when response to laxatives has not been sufficient. In a study of 14 healthy volunteers evaluating the use of morphine plus oral methylnaltrexone in increasing doses, methylnaltrexone significantly reduced morphine-induced delay in oral-cecal transit.[27] Another study reported subcutaneous methylnaltrexone is effective in inducing laxation in patients receiving palliative care who have opioid-induced constipation and in whomconventional laxatives have failed.[28] However, because methylnaltrexone has only recently been approved by the US Food and Drug Administration (FDA), more rigorous trials are needed.
- Alvimopan is indicated to help prevent postoperative ileus following bowel resection. It has a longer duration of action than methylnaltrexone. Taguchi et al examined 78 postoperative patients randomized to receive either placebo or alvimopan.[29] Fifteen patients underwent partial colectomy, 36 were status post simple hysterectomy, and the remaining 27 underwent radical hysterectomy. All of the patients were on patient-controlled analgesia pumps using either meperidine or morphine. Compared with patients on placebo, patients on alvimopan had their first bowel movement 2 days earlier, resumed a solid diet 1.3 days earlier, and returned home 1.4 days earlier. Other recent trials have been completed, including a meta-analysis comparing alvimopan with placebo[30] and a study that found alvimopan to accelerate gastrointestinal tract recovery after bowel resection, regardless of age, gender, race, or concomitant medication.[31]
- Alvimopan (Entereg): Peripherally acting mu-opioid receptor antagonist. Binds mu-opioid receptors in gut, thereby selectively inhibiting negative opioid effects on GI function and motility. Indicated for postoperative ileus following bowel resection with primary anastomosis. Five clinical studies with enrollment >2500 patients demonstrated accelerated recovery time of upper and lower tract GI function with alvimopan compared with placebo. Decrease of hospital days also observed in alvimopan group compared with placebo. Only available to hospitals after they complete a registration process designed to maintain the benefits associated with short-term use and prevent long-term, outpatient use (Entereg Access Support and Education [EASE] program).
- Use of prokinetic agents has shown mixed results. Randomized trials have shown some benefit of the colon-stimulating laxative bisacodyl for the treatment of ileus.[32, 33] Erythromycin, a motilin receptor agonist, has been used for postoperative gastric paresis but has not been shown to be beneficial for ileus.[34] Metoclopramide (Reglan), a dopaminergic antagonist, has antiemetic and prokinetic activities, but data have shown that the drug may actually worsen ileus. In a randomized controlled study on 210 patients undergoing major abdominal surgery, Wattchow et al reported that perioperative low dose celecoxib markedly reduced the development of paralytic ileus compared to diclofenac.[35] The effect was independent of narcotic use and was not associated with any increase in postoperative complications.
- A review of meta-analyses and randomized controlled trials on drugs used for post-operative ileus was reported by Yeh et al.[36] The investigators identified three meta-analyses (2 on gum-chewing and 1 on alvimopan) and 18 clinical trials. Only gum chewing and alvimopan were effective in preventing ileus but due to safety concerns and costs with alvimopan, gum chewing may be preferred as first-line therapy. Gum chewing has also been used in women recovering from cesearian section with good effect when compared to standard of care in a randomized study conducted.[37]
